What ions are in an aurora?
Auroras occur between 80 and 250 km in altitude in the thermosphere, which contains regions D,E, and F of the ionosphere. There are 14 major species of ions involved in auroras. The main ions are O+, NO+, and O+2. The main uncharged gases are nitrogen (N2) and monoatomic oxygen (O).
The reason there is so much monoatomic oxygen is that 33% of the absorbed radiation in the thermosphere goes to splitting O2 molecules into O atoms.
Cosmic rays, protons (H+ ions), and He+ ions play almost no role in auroras. Those few protons that exist in the ionosphere are produced ‘accidentally’ by chemical reactions such as resonant charge exchange with O+ ions.[1]
| Region | Main neutrals | Main ions | Degree of ionization |
|---|---|---|---|
| Exosphere (690–10,000 km) | H+, He+ | ||
| Ionosphere | |||
| F region (150–690 km) | O, N2, He | O+ | 10−3 |
| E region (90–150 km) | O, N2, O2 | O+2, NO+, N+2 | 10−6 |
| D region (48–90 km) | N2, O, O2 | ||
| Mesopause (85 km) | |||
| Mesosphere (50–85 km) | N2, O2 | ||
| Stratosphere (20–50 km) | N2, O2, O3 | ||
| Troposphere ( 0–20 km) | N2, O2 |
The F layer is divided into four layers: F1, F2, F3, and topside ionosphere (600–1500 km). Other minor layers include the He layer at the O+ to H+ transition at the top of the F region, the protonosphere (>1500 km), where the predominant ion is H+, and the sporadic E layer, which is a very narrow layer less than 2 km wide below the E layer. The sporadic E layer contains metals such as Fe+ and Mg+.
The D layer is complex. According to the SIC model, it contains N2, O, O2, O3, N, NO, NO2, CO2, and H2O as well as 24 positive ions and 11 negative ions including exotic ions like CO−4 and O−4 and 174 chemical reactions.[2]
What causes the aurora?
Aurora is caused by primary photoelectrons produced by solar UV, and by secondary auroral electrons produced in reactions in the upper atmosphere. These reactions raise the gas to an excited state which then spontaneously decays [2], releasing photons at specific wavelengths, known as emission lines. The emission lines are extremely narrow because the air pressure in the ionosphere is so low.

Aurora at 38° latitude showing green and red emission (5577 and 6300 Å) from monoatomic oxygen
Why do electrons, which are negative, produce positive ions?
You might think that since electrons are the main drivers of auroras they would produce negative ions. However, the vast majority are cations (positive ions). Primary electrons produce positive ions by splitting N2 and O2 molecules into N+, O+, N, and O. This releases secondary auroral electrons, which balance the charge in the reaction. Another reaction called photodissociation occurs in which gases are split without producing ions.
A typical reaction is photoionization [1], where a primary electron (e−p) is absorbed by a molecule or atom M, creating an ion and a secondary auroral electron (e−s):
e−p + M → e−p + M+ + e−s
Singlet oxygen (which means no unpaired electrons, see below) is important in auroras. For example, nitrogen can react with monoatomic oxygen in the reaction
N2(A3Σ+u) + O → N2(X1Σ+g) + O(1S0)
producing a ground state nitrogen and singlet monoatomic oxygen. According to M. H. Rees [1], this chemical reaction is the principal source of O(1S0) for the oxygen green line.
Another way O(1S0) is produced is by electron impact with ionized oxygen:
O+2 + e− → O + O(1S0).
Chemical reactions
Chemical reactions are also important for auroras. Over 100 have been described. An example is nitric oxide ion made from reaction of nitrogen ion with monoatomic oxygen:
N+2 + O → NO+ + N(4S)
which gives off a blue photon at 4025 Å.
Radiative Recombination
Another source of light in airglow and possibly auroras is radiative recombination (RR), for example:
O+ + e− → O
which emits at 8446 [7] and 7774 Å [8] in the near infrared. This reaction is very slow in the ionosphere. O actually gets its electron in a two-step process called dissociative recombination (DR), where atoms change hands by dealing with an broker such as nitrogen: [10]
O+ + N2 → N + NO+
NO+ + e− → N + O
This reaction is nearly five orders of magnitude faster than direct recombination.
RR is a two-step process whereby two ions combine, leaving a neutral atom in an excited state. The neutral atom then relaxes to the ground state, releasing a photon. Figuring out the mechanism isn't easy, as an excited atom can be produced in a variety of ways. An atom doesn't care where it gets its energy from. Another example of RR is the famous HIα line (6563 Å) found in interstellar nebulae.
An example of DR is the ground state dissociation of O+2 into neutral oxygen atoms:
O+2 (X2Πg) + e− → O(1D) + O(1D)
which emits a UV photon along with 3.06 eV excess energy which goes to heat.[2]
Negative ions
As mentioned above, negative ions are rare in the ionosphere but can be formed in the lower atmosphere. There's a whole industry out there claiming that negative ions, mainly hydrated ∙O−2 and CO−3 produced by corona discharge, are good air fresheners. Unfortunately, the health benefits as well as the light they emit are hard to detect.
If an energy state is above the ionization limit, it's not quantized and its emission is not a single line but a continuum that extends into the short wavelength region. This is common with negative ions in stellar atmospheres where the abundant H− ion on the sun releases its extra electron and turns into neutral H.
H− + hν → H + e− + leftover energy
The threshold of photoionization is 1.65 μm, in the infrared [9], so any photon (written as hν) with that wavelength or shorter will work. Of course a star has lots of those.
Transitions
What's most interesting about auroras is not the states, but transitions between states. Some of these reactions are written in spectroscopic notation, which I'll describe below. Don't panic, it's very simple. It looks like math, but it's not, I swear.
An atom or molecule can lose energy by emitting a photon or by colliding with another molecule, which generates heat. Whether we get a beautiful emission line or just ordinary, boring heat depends on the decay rate and the concentration of other molecules. If the excited state decays rapidly, we're more likely to see a stronger auroral line because there is less chance of colliding with another molecule or atom. This is one reason auroras and airglow are above the tropopause at 85 km, where the air is much thinner.
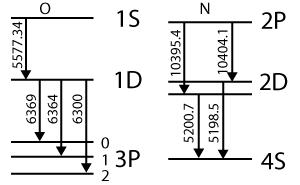
O and N transition lines. Emitted wavelengths are in Angstroms. Longer line = higher energy = shorter wavelength (Redrawn from [2], p.233)
The brightest visible line in an aurora is the oxygen green line at 5577 Å (Angstroms). The green is due to a transition of a singlet oxygen O(1S0) to a lower energy state 1D2. Three closely spaced red lines (6300, 6364, and 6369 Å) are emitted when the O atom drops from the 1D2 state to its ground triplet state, O(3P2,1, or 0) (see diagram at right). The 'red' and 'green' transitions can also occur simultaneously in one single jump from O(1S0)→O(3P1). This gives off UV light at 2972 Å, which can't be seen from the ground.
Nitrogen also undergoes spontaneous de-excitation. The jump from 2P to 2D is in the IR, close to the 1 μm wavelength limit for a silicon CCD. The jumps from 2D to the ground state (4S) would be green. Whether these emissions are visible depends on the relative rates of collision and spontaneous de-excitation, and of course on the amount of N.
If an excited atom or molecule doesn't emit a photon immediately, it's said to be in a metastable state. For example, the O(1D) state, which can decay by emitting red lines at 6300 Å, has a half-life of 100 seconds and so has a greater chance of colliding with something. That's why it's fainter than the green line from decay of O(1S0) singlet oxygen. O(1S0) is also metastable but lasts for only 0.91 seconds before emitting a photon. Metastable states can only survive in a low density thermosphere.

Aurora with Ursa Major in the background
These strong aurora lines, including the red triplet and the green O line at 5577 Å, are metastable because the transitions are “forbidden”, which means they break the selection rules that determine whether a transition is allowed to happen. Breaking the laws of physics has a price: the light from these rebels without a cause is much fainter than it should be.
Blue
Dinitrogen ion (N+2) has a faint transition in the blue region that is sometimes observed, but it is often hard to distinguish from the blue caused by scattering of moonlight. Some textbooks [11] maintain that ozone contributes to the blue color of the sky, but it is only significant when the Sun is well below the horizon. They say this is due to absorption of red light in the Chappuis bands at 575–603 nm, and they have calculations that support it.

Narrow red aurora-like phenomenon resembles a STEVE (Strong thermal emission velocity enhancement) [12] but is actually a stable auroral red arc [13] (SAR) at 38° latitude during the Oct. 10, 2024 aurora (9:15 pm). This one was almost directly overhead, far to the south of the main aurora. It stretched from horizon to horizon and was nearly undetectable to the eye. Camera is facing east
Stable auroral red arcs
In stable auroral red arcs, monoatomic oxygen O(3P) is excited to singlet oxygen O(1D). O(1D) undergoes spontaneous de-excitation back to the triplet O(3P) state, emitting a red oxygen line at 6300 Å (630 nm). They are typically at higher altitude (≈ 400 km) than a diffuse aurora (≈ 200 km).
Spectroscopic notation
If you read any papers on this subject, you'll encounter sentences like this:
There is a collisional coupling between the B3Πg state and triplet A3Σ+u, W3Δ+u, B′3Σ−u states.
This is a shorthand notation for the energy levels in a molecule. The Greek letters and subscripts are called molecular term symbols. To read any good articles on auroras, you need to know this notation.
For atoms: Atom (2s+1LJ) Example: O(1D2)
For diatomic molecules: Molecule (State 2s+1Λ (+/−)Ω,(g/u),v=0 ) Example: N2(A3Σ+u)
The rules are: [3]
The main symbol (L or Λ) is the total orbital angular momentum. Latin letters are used for atoms and Greek letters are used for molecules. For historical reasons, a specific sequence of letters is used:
Orbital momentum 0 1 2 3 4 5 6 ... Atoms (L) S P D F G H I ... Molecules (Λ) Σ Π Δ Φ Γ H I ... Upper case letters are always used for whole atoms. Lower-case is reserved for individual electrons or orbitals.
J is a quantum number for angular momentum.
The 2s+1 superscript on the left is the multiplicity, which is 2 × total spin number + 1. There are a maximum of 2 electrons in an orbital. Spin number is determined by whether they are paired. If they are, we have singlet (total spin S = 0) ↑↓. Otherwise we might have a doublet (1 unpaired electron, total spin S = ½) ↑↑↓, a triplet (2 unpaired electrons, total spin S = 1) ↑↑, ↑↓, ↓↓, or some higher number.
Terms like ‘singlet’ and ‘doublet’ don't come from the number of unpaired electrons. They come from the number of lines in the spectrum: a singlet means it has a single line, a triplet has three lines, and so on.
Don't confuse this with the notation for atomic isotopes, which also has a superscript on the upper left.

A xenon calibration source generates many emission lines at wavelengths from UV to visible while giving sage advice at the same time.
g / u is the parity (for homonuclear diatomic molecules). Don't worry about this; no one really cares about parity.
The + / − superscript is not electric charge, but the reflection symmetry in the plane of the molecule. Obviously, this isn't needed for atoms, only molecules.
The subscript on the right (Ω) is the total angular momentum, i.e. Λ + magnetic spin. Since this is somewhat redundant, it's not always used. It is some multiple of ½.
For the state, X is the ground state, and excited states are labeled A, B, C, D, etc. in order of increasing energy. Capital letters are used if their multiplicity is the same as the ground state, and a, b, c, d otherwise. Molecular nitrogen is unique in having an extra state between A and B, called W [4] apparently for historical reasons. It is said to be the fourth lowest electronic state in N2.
Also sometimes seen is Δ𝑣, which is a change in vibrational state. Molecular vibrations are small compared to electronic energy levels and aren't always reported. The Δ subscript is not to be confused with the Δ angular momentum term. They have totally different meanings.
The terms are often abbreviated. You might see the red oxygen line just written as O1D. This means the line is caused by O(1D2) state going to the triplet ground state, which is O(3P2,1,or 0).
How do people know which lines correspond to which transitions?
Researchers can compare spectrographic recordings from auroras with artificial line sources like the one at right. Since many auroral lines are in the far UV, some recordings can't be made from the ground. Measuring spectra from the ground is made more challenging due to light pollution, especially from mercury and sodium vapor lamps, and by the vast numbers of lines in the infrared from water vapor in the atmosphere. Many emission lines overlap, so complicated calculations are sometimes needed to discriminate between alternative assignments.[5]
Do auroras emit ultraviolet and infrared light?
Jupiter is not the only planet that has a UV aurora. There are many strong UV auroral emissions from Earth's ionosphere as well, such as the OII line at 833–834 Å, a wavelength far too short to be observed from the surface. About 71% of Earth auroral lines are in the UV, 20% are in the visible, and 9% are in the infrared.
Close-up of xenon calibration source
Night airglow also contains a large number of lines from O2 in the UV as well as a weak continuum throughout the visible region.[1] This means it would be challenging to detect near-UV emissions from the ground even with a UV-sensitive camera.
Many other UV emission lines have been found in auroras, including the 538 Å line from OII and the H Lyman-α and -β lines (1215.7 and 1025.72 Å). The H Lyman-α and -β lines are caused by resonance scattering of solar UV radiation [1] and aren't normally considered part of an aurora. There are also infrared lines from vibrational states of O2 as far out as 147 and 63 μm.
The HIα line (6563 Å) in the far red, well known to astronomers but barely detectable to the eye, is a major component of airglow, along with many emission lines in the near infrared from OH, which contribute to sky brightness. These OH lines are considered a major nuisance by astronomers, who have built sophisticated fiber Bragg filters to get rid of them.
Near-IR emissions from 7320 to 7330 Å due to forbidden transitions in OII and emissions at 6548 and 6583 Å from NII have also been observed in auroras. These could be detected by modified DSLR cameras.
Common visible (400–700 nm) aurora wavelengths
| Color | Wavelength (nm) | Transition | Ref. |
|---|---|---|---|
| 427.8 | N+2 (B2 Σ+u → X2 Σ+g) (0–1) | 6 | |
| 434.9 | O+(4P01/2-5/2) → O+(4P1/2-5/2) | 1 | |
| 436.8 | O(3P2,1,or 0) → O(3S01) | 1 | |
| 441.6 | O+(2D03/2,5/2) → O+(2P1/2,3/2) | 1 | |
| 464.9 | O+(4D01/2-7/2) → O+(4P1/2-5/2) | 1 | |
| 493.5 | N(2S01/2) → N(2P1/2,3/2) | 1 | |
| 496.8 | O(5D4-0) → O(5P3,2,or 1) | 1 | |
| 500.1 | N+(3F04,3,2) → N+(3D3,2,1) | 1 | |
| 500.6 | N+(5P03,2,1) → N+(5P3,2,1) | 1 | |
| 504.5 | N+(3S1) → N+(3P02,1,0) | 1 | |
| 520.0 | N(2D03/2,5/2) → N(4S3/2) | 1 | |
| 557.7 | O(1S0 → O1D2) | 6 | |
| 567.9 | N+(3D3,2,1) → N+(3P02,1,0) | 1 | |
| 570–610 | N2(A3 Σ+u → B3 Πg Δv=4) | 6 | |
| 575.5 | N+(1S0) → N+(1D2) | 1 | |
| 630.0 | O(1D2) → O(3P2) | 6 | |
| 636.4 | O(1D2) → O(3P1) | 6 | |
| 636.9 | O(1D2) → O(3P0) | 6 | |
| 630–680 | N2(A3 Σ+u → B3 Πg Δv=3) | 6 | |
| 632.3 | N2(B3Πg Δv=10) → N2(A3Σu Δv=7) | 5 | |
| 639.5 | N2(B3Πg Δv=9) → N2(A3Σu Δv=6) | 5 | |
| 646.9 | N2(B3Πg Δv=8) → N2(A3Σu Δv=5) | 5 | |
| 654.5 | N2(B3Πg Δv=7) → N2(A3Σu Δv=4) | 5 | |
| 658.3 | N+(1D2) → N+(3P2,1,0) | 1 | |
| 662.4 | N2(B3Πg Δv=6) → N2(A3Σu Δv=3) | 5 | |
| 670.5 | N2(B3Πg Δv=5) → N2(A3Σu Δv=2) | 5 | |
| 678.9 | N2(B3Πg Δv=4) → N2(A3Σu Δv=2) | 5 | |
| 687.5 | N2(B3Πg Δv=3) → N2(A3Σu Δv=0) | 5 | |
| 777.4 | O+(4S) + e− → O* | 8 | |
| 844.6 | O+(4S) + e− → O* | 7 |
Colors are approximate, based on CIE equivalents. Not all emission wavelengths have the same intensity. Bold = stronger
Why is any of this important?
1. It's always nice to know what you're looking at, besides admiring the pretty colors.
2. It tells us that matter is quantized. None of this would be possible without quantum mechanics. Emission lines were historically important in the realization that energy is quantized. When you watch an aurora, you're seeing quantum mechanics in action.
3. According to the rules of chemistry, watching an aurora is watching 'forbidden' light. What could be more exciting than that?
Why doesn't the government tax auroras?
Oh, give it time, baby.
[1] Rees MH. Physics and chemistry of the upper atmosphere. Cambridge, 1989.
[2] Schunk R, Nagy A. Ionospheres: Physics, Plasma Physics, and Chemistry, 2e, p. 233 and 355.
[3] Svanberg S. Atomic and molecular spectroscopy, 4e. Springer, 2004.
[4] Benesch WM, Saum KA (1971). The W 3Delta u state of molecular nitrogen. J. Phys. B: Atom. Mol. Phys. 4 732 DOI 10.1088/0022-3700/4/5/016. paywalled.
[5] Kirillov AS (2008). Electronically excited molecular nitrogen and molecular oxygen in the high latigude upper atmosphere. Ann Geophys. 26, 1159–1169 www.ann-geophys.net/26/1159/2008/
[6] Barthelemy M, Robert E, Kalegaev V, Grennerat V, et al. (2022) AMICal Sat: A sparse RGB imager on board a 2U cubesat to study the aurora. IEEE Journal Miniaturization Air Space Systems. arxiv:2201.06973v1 [astro-ph.IM]
[7] Waldrop L, Kerr RB, Huang Y (2018). Evidence for Radiative Recombination of O+ Ions as a Significant Source of O 844.6 nm Emission Excitation. Journal of Geophysical Research: Space Physics, 123, 3078–3086. https://doi.org/10.1002/2017JA024790
[8] Oyama S, Tsuda TT, Hosokawa K,Ogawa Y, et al. (2018). Auroral molecular emission effects on the atomic oxygen line at 777.4 nm. Earth, Planets and Space 70:166. https://doi.org/10.1186/s40623-018-0936-z
[9] Thorne AP, Spectrophysics, 2e. Chapman and Hall, 1988, p.368
[10] Schunk R, Nagy A. Ionospheres: Physics, Plasma Physics, and Chemistry, 2e, p. 243.
[11] Bohren CF, Clothiaux EE (2006). Fundamentals of Atmospheric Radiation: An Introduction With 400 Problems, p 409–415. Reviewed here.
[12] Gallardo-Lacourt, Liang J, Nishimura Y, Donovan E (2018). On the origin of STEVE: particle precipitation of ionospheric airglow? Geophys. Res. Lett. 45 (16), 7968–7973 Link
[13] Mendillo M, Baumgardner J, Wroten J (2016). SAR arcs we have seen: Evidence for variability in stable auroral red arcs. J. Geophys. Res. 121 (1), 245–262. Link
may 23 2024, 4:18 am. last updated oct 13 2024
