 avid Gortner, who has a doctorate in pharmacy, has an
article
over at PJmedia blasting the FDA for granting EUAs to the two new COVID
drugs (Molnupiravir and Paxlovid) while ignoring epidemiological evidence
for HCQ and Ivermectin.
avid Gortner, who has a doctorate in pharmacy, has an
article
over at PJmedia blasting the FDA for granting EUAs to the two new COVID
drugs (Molnupiravir and Paxlovid) while ignoring epidemiological evidence
for HCQ and Ivermectin.
I happen to think that granting an EUA to molnupiravir and refusing to allow ivermectin are terrible decisions. But there's a difference. Paxlovid is a combination of nirmatrelvir and ritonavir, and as with molnupiravir its mechanism of action is understood. Nirmatrelvir, aka PF-07321332, is clearly a peptide derivative, which makes sense for a protease inhibitor. Molnupiravir is a nucleotide analogue that screws up RNA replication. By contrast, the two populist drugs, ivermectin and HCQ, have two things in common: they are mired in controversy, and nobody knows how they work.
This is no coincidence. Sure, we have hypotheses: HCQ might work by changing the pH of the lysosome, thereby inhibiting the viral protease. Or it might be like ivermectin, which is said to prevent the virus from blocking the Type-I interferon response. The problem with both drugs is that the concentrations where these wonderful things happen aren't achieved by the usual dose.
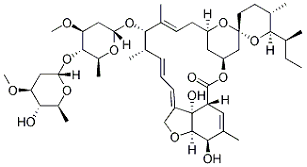
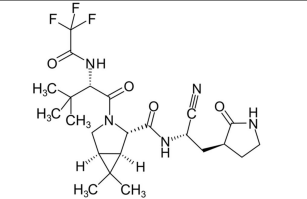
Top: Ivermectin structure. Bottom: Nirmatrelvir
If you don't know the target or the mechanism of action, what do you do? Do you give it early in moderate doses as a prophylactic course or as a single large bolus at the peak of the infection? What concentration of the drug in the blood plasma or in the target organ should you aim for? If you don't even know what the drug is supposed to do, how do you even evaluate efficacy?
The answer is, you can't. And increasingly, that's just fine with drug manufacturers and medical practitioners. It's a widespread attitude in modern medicine: it doesn't matter how something works; all that matters is whether the patient gets better. That sounds reasonable, but it simply doesn't work. Not knowing how a drug works is a recipe for failure. I've seen it firsthand: many drugs fail in clinical trials not because they don't work, but because they were rushed to the clinic before we knew how they worked.
Let's get engaged
You don't just want to know the mechanism of action for fun. It's necessary before you can measure target engagement. For instance, if the drug is supposed to travel to the nucleus and preserve the interferon response, you have to measure the concentration of your drug in the nucleus of cells from your patients to show that it gets there. Then you have to measure the amount of interferon that gets released.
Well, technically you don't have to, but if you don't, you have no way of knowing why the drug didn't work. You'd just be throwing the dice, and you'd be back at square one when it fails. It would be almost as if you didn't care whether it worked or not. Maybe you accidentally used the wrong drug. Or maybe, as in some clinical trials, you don't want the drug to work at all.
There are a million ways to screw up an experiment, and a million reasons why people might do it, and that's what a drug trial really is: an experiment. Not knowing the target of the drug is one of the best ways to screw it up.
Warm and fuzzy feelings
The idea is that we need a treatment now and can't wait for scientists to figure out how it works; all that matters is whether it works. A related issue is the trend of dropping the control and placebo groups in a drug trial on the theory that if the drug works, you're condemning the placebo patients to death.
That idea may convince patients that you have their best interest at heart, but it is perilously close to moral grandstanding. The purpose of a clinical trial is not to cure patients. The subjects know this, and their courage in facing that fact is why we admire them so much. Its purpose is to determine whether your drug benefits them or harms them. That might not give you that warm fuzzy feeling that people crave, but it is essential knowledge, and by short-circuiting the process we end up with what we have now with Ivermectin and HCQ: accusations of politics, greed by pharma companies, and corruption at the FDA, and we still don't know whether the drug is any good.
Ivermectin and HCQ may indeed be victims of politics, greed, and corruption. And there are some dishonest scientists out there. But the system of science, drug testing, and the approval process was invented to compensate for these factors. If everyone was perfectly honest, we wouldn't need a system at all. I could just say “my drug cures COVID” and people would believe me and start using it and everyone would live happily ever after. Unfortunately, that warm fuzzy feeling runs into the brick wall of hard logic that says if the drug doesn't get to the target, all the statistical analyses in the world won't help it cure a disease.
Statistics tell you that something might be happening. Mechanistic biology, when it works, tells you cause and effect.
Big Pharma and Alzheimer's disease
Now on to a topic that's a lot more interesting.
Last week I got into a discussion with a colleague about ApoE4, a protein that is the biggest genetic risk factor for late-onset Alzheimer's disease. ApoE4 carriers have a 7.5% chance of getting AD by age 85 and a 35% chance by age 95. About 40% of people with AD have either one or two alleles of ApoE4, but AD is rare in people with ApoE2—the ‘good’ variant.
Why that should be so is unknown. ApoE4 could be toxic or it may simply be deficient in its protective function, or it could be both. The ‘deficiency’ theory is supported by the fact that when a patient gets traumatic brain injury, large amounts of ApoE are expressed, which provide the neurons with the cholesterol they need to repair themselves. Maybe, the theory goes, ApoE4 can't carry cholesterol as well, or maybe it can't pass through the membrane proteins, called receptors, that bring ApoE4 and the precious cholesterol cargo into the cell. There's also evidence for a ‘toxic gain of function’ theory.
My colleague said it didn't matter which theory was correct. He suggested giving tamoxifen to AD patients to block the expression of ApoE.
Tamoxifen
Tamoxifen is a breast cancer drug that inhibits ApoE expression by blocking the estrogen receptor, or ER, in the brain. Upon stimulation with estradiol, the ER causes the cell to produce more ApoE.
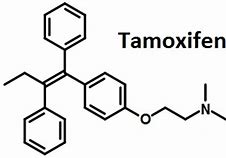
Tamoxifen structure
In a dense paper that came out last year, Wang et al.[1] used tamoxifen to block expression of ApoE in transgenic mice and found that it reduced tauopathy and tau-mediated neurodegeneration. Tau protein closely parallels neurodegeneration. Whether it actually induces it or is caused by it is not yet known. But the appeal of this idea is that we don't have to know why ApoE4 is a risk factor. We just block it and see what happens.
Mice can survive without ApoE. The only symptom we can observe is extremely high cholesterol, which could cause cardiovascular problems in humans. So if we gave people tamoxifen, we'd also have to give them intravenous injections of ApoE once a week or so. This ApoE would not cross the blood-brain barrier, and it would help reduce the side effects of tamoxifen. Tamoxifen would have to be given continuously for decades, starting at around age 65, to healthy patients, and the results would not be known for years. This is probably one reason no one has ever done a clinical trial, and there's no doubt it would be a ruddy gold mine for Big Pharma.
But there's a problem. Sex hormones, including estradiol, have different functions in the brain than in the periphery. Estrogens affect the hypothalamus and pituitary. They are important for affective state, vulnerability to epilepsy, attention, pain sensitivity and even motor coordination. Estrogen has an anti-inflammatory effect and it induces excitatory synapse formation in the hippocampus, indicating a powerful effect on memory. Blocking estrogen in the brain could make the patients worse.
Way back in 2001, a non-steroidal tamoxifen-like drug called CI-628 was found to block estrogen-induced synapse formation. This is very bad, because synapse formation is how we form memories. Anti-androgens, often given to prostate cancer patients, are also said to lead to an increased risk of AD.
As much as drug companies would love to give us an expensive drug (it probably wouldn't be tamoxifen, but something proprietary and very, very dangerous) that 20% of the population would have to take every day for thirty years, somebody somewhere is going to have to figure out why not everybody with an ApoE4 gets AD and what happens to them when the ER is blocked. And that means somebody is going to have to pay for it.
Ultrasound becoming quiet
Here's another example of how not knowing how a treatment works leads to failure.
A few years ago, there was some excitement among some AD researchers about using ultrasound to blast open the blood-brain barrier. It was based on the theory that the reason beta-amyloid accumulates in the brains of AD patients might be a failure of ‘clearance,’ which is to say beta-amyloid is not being removed from the brain. If so, it was thought, beta-amyloid could build up to toxic levels.
There is some evidence that the blood-brain barrier, which is a layer of cells around the blood vessels throughout the brain, becomes dysfunctional in AD. Some reports find that the BBB permeability decreases in early AD, but now it is increasingly accepted that BBB leakage is a sign of cognitive decline.[2] If so, ultrasound would make the patients worse.
Despite an unconvincing early paper, this topic has been very quiet for several years, suggesting that blasting patients with sound waves didn't work. We also know from the failed big pharma clinical trials that eliminating beta-amyloid from the brain has no effect on the course of the disease, suggesting that whatever beta-amyloid is doing, it is not the direct cause.
Running long-shot experiments that fail is not without costs. Several years ago the Gladstone Institute in California tried to develop “corrector” drugs that would bend the ApoE4 protein into the right shape to mimic ApoE2. They didn't work, and now anyone with a similar idea will have great difficulty getting funded. Anyone who proposes ultrasound, maybe a different frequency or intensity, will now have to deal with the fact that ultrasound was tried and it didn't work. It's not an impassable barrier, but if you're a grant reviewer it gives you a reason to say no, and that's all it takes.
1. Wang C, Xiong M, Gratuze M, Bao X, Shi Y, Andhey PS, Manis M, Schroeder C, Yin Z, Madore C, Butovsky O, Artyomov M, Ulrich JD, Holtzman DM. Selective removal of astrocytic APOE4 strongly protects against tau-mediated neurodegeneration and decreases synaptic phagocytosis by microglia. Neuron. 2021 May 19;109(10):1657-1674.e7. doi: 10.1016/j.neuron.2021.03.024. PMID: 33831349; PMCID: PMC8141024.
2. Nehra G, Bauer B, Hartz AMS. Blood-brain barrier leakage in Alzheimer's disease: From discovery to clinical relevance. Pharmacol Ther. 2022 Jan 30:108119. doi: 10.1016/j.pharmthera.2022.108119. PMID: 35108575.
feb 11 2022, 5:44 am. updated feb 14 2022
