 erhaps the most surprising thing about vaccines is the people who
thought the vaccine would put an end to COVID. They seem to have
believed that it's possible to produce a vaccine against a coronavirus
that doesn't lose effectiveness after a few months. So far it appears
that it's not.
erhaps the most surprising thing about vaccines is the people who
thought the vaccine would put an end to COVID. They seem to have
believed that it's possible to produce a vaccine against a coronavirus
that doesn't lose effectiveness after a few months. So far it appears
that it's not.
The Vaccination scenario
Coronaviruses mutate far too rapidly for a vaccine to be effective for long, which is why we have never had a vaccine against the common cold. At best, a vaccine will buy time to develop and test new antiviral drugs that can treat the disease.
The media are trying to blame the unvaccinated for creating new COVID variants. This is not backed by science. New variants form spontaneously. It is the antibodies—whether formed during an infection or after a vaccine—that create the evolutionary pressure for them to spread.
The value of the vaccine is to buy time until an antiviral drug is found, or until the virus spontaneously mutates into something harmless.
There are many good reasons to get vaccinated, but fear of creating new variants is not one of them.
To prevent SARS-CoV-2 using vaccines we would need “boosters” every few months. This is precisely what's happening now, with the vaccines showing reduced protection from delta and lambda variants. This will only get worse as the flu season approaches.
Most vaccines are designed to produce antibodies against the S1 subunit of the SARS-CoV-2 spike protein. The authors of a paper published last week in Science explain why this is a problem:
The S1 subunit is the major target of (neutralizing) antibodies (Abs), and is more genetically variable than the S2 subunit (2–5). Accordingly, Abs binding to the S1 subunit receptor-binding domain (RBD) and N-terminal domain (NTD) exert a selective pressure resulting in the emergence of new variants (5–10).[10]
The authors discovered that some COVID patients can produce antibodies against the S2 subunit, which mutates at a lower rate. This suggests that if we could raise an antibody against some portion of the virus that cannot be mutated without losing infectiousness, it might be possible to create a longer-lasting vaccine. It won't be easy: the antibodies had low abundance because the S2 region is partially hidden inside the virus protein. (Incidentally, that article is paywalled, suggesting that the time of free COVID articles is now over. That's the best evidence yet that we're no longer in a pandemic.)
Even so, many people think resources should be redirected into small molecule drugs produced in the more traditional way. Unfortunately, to avoid the lengthy and inefficient FDA approval process, which only seven to ten percent of drugs survive, we're currently limited to repurposing existing drugs. Over 1,900 different drugs have been reported to be effective in vitro and promoted as candidates for possible clinical trials. To date, 316 clinical trials (180 of hydroxychloroquine [HCQ] and 133 of the 32 others) have been conducted on 33 of these drugs, with disappointing results. A recent large study found that remdesivir, HCQ, liponavir/ritonavir, and interferon-β1a have all failed in reducing hospital stay, mortality, and need for ventilation.[1] Arbidol (umifenovir) has also failed in repeated tests.[2]
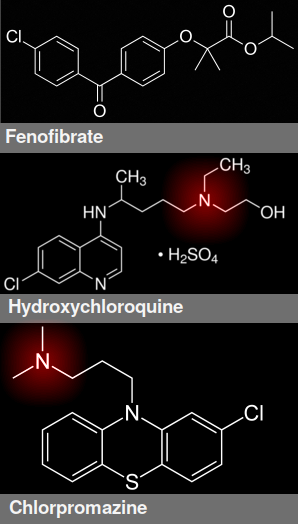
Structure of fenofibrate, hydroxychloroquine, and chlorpromazine. Cation is highlighted in red
The Diabetes gambit
The high mortality among diabetes patients has led numerous investigators to suggest that lipid metabolism is important in COVID-19 pathogenesis. One study found that drugs targeting VPS34 (a class III phosphatidylinositol 3-kinase, an enzyme involved in triacylglycerol production) and protein palmitoylation (in which the cell attaches a lipid to the protein to tether it to the membrane) impaired SARS-CoV-2 replication.[3] The theory is that coronaviruses reorganize cell membranes to form replication organelles.
The diabetes drug fenofibrate, which activates a ligand-activated transcriptional factor called PPARα (peroxisome proliferator activated receptor alpha), is also being hyped in the press. Activating PPARα increases the activity of lipoprotein lipase, causing a decrease in triglycerides and LDL levels, and modestly raises HDL by 5 to 20%.[4]
Another theory is that SARS-CoV-2 upregulates expression of HMG-CoA synthase and squalene monooxygenase (enzymes involved in cholesterol biosynthesis) because it stimulates the need for lipid rafts and palmitoylation of viral proteins that are essential for viral replication.[5] When cholesterol accumulates in macrophages, pneumocytes, and other immune cells in the lung, it causes inflammation.[6,7]
That's the theory. As interesting as these findings may be, some pharmacologists argue that the high drug concentrations needed are a red flag that the drug is acting through a nonspecific effect. Remdesivir, for example, has an IC50 of 1.5 micromolar, which is considered high (the lower the IC50, the more potent the drug). Other drugs, including triacsin C (which inhibits acyl CoA synthase) and orlistat (which inhibits fatty acid synthase), have IC50s of 20 and 500 micromolar, respectively. Such high concentrations mean that much drug development work would be needed to turn them into useful drugs.
The Tummino affair
Many other repurposed drugs, including vitamin D, SSRIs, and HCQ, have been suggested, mainly on the basis of in vitro studies. Last week a study of 310 such drugs, published in Science, claimed that their antiviral effects are all artifacts. Tummino et al.[8] found that all cationic amphiphilic drugs accumulate in the endosomes and lysosomes and interfere with phospholipid production. The disruption of lysosome and endosome compartments changes the pH, which is said to reduce viral entry and propagation by virtue of the pH-dependence of enzymes.
The most effective of these are amiodarone and chloroquine, but many other drugs such as chlorpromazine and tamoxifen have all been shown to have in vitro efficacy against the virus. The authors state that these are all false positives—what scientists call a nonspecific effect—because the effect usually occurs above 100 nanomolar, which means that high doses are needed. Specifically, say Tummino et al., the drugs are causing drug-induced phospholipidosis.
Drug-induced phospholipidosis is a physical effect that occurs in the lysosome. It's caused by any drug that has a cationic amphiphilic motif, which is a positive charge attached to a lipid-soluble and water-soluble domain.[9] The result is an accumulation of phospholipids and lamellar bodies, which are subcellular organelles that act as pulmonary surfactants (i.e. detergents), and it's typically associated with adverse drug reactions. Any drug that binds phospholipids can cause phospholipidosis.
Indeed, this was how HCQ was originally said to work. Azithromycin also induces phospholipidosis, suggesting an explanation why the two drugs could have produced a synergistic effect.
Many people think of the lysosome as the rubbish bin of the cell. That would be incorrect. Lysosomal enzymes called cathepsins are crucial in controlling mitosis, cell motility, and antigen presentation. Lysosomal autophagy, where the cell digests part of itself, is an important alternative to apoptosis. And it just may be that creating pulmonary surfactants is helpful in getting rid of viruses. Understanding what's happening will help inform dosing—an important issue because when and where the drug is given may determine whether it works or not.
The Covid Caper
The authors showed a sigmoidal correlation between phospholipidosis and suppression of viral loads in cultured cells. However, none of the drugs showed any effect in mice. Only remdesivir reduced viral load in mice (by 1000×). The use of a mouse model is a possible weakness, as humans and mice may react differently. The weakness of using mice is also shown by the recent NEJM paper showing that remdesivir doesn't work in humans.[1]
These results don't mean that drugs that act by inducing phospholipidosis don't work. Indeed, their own results show that inducing phospholipidosis would be a viable strategy if the cell culture results could be translated into humans. What they should have said is that if drugs like amiodarone, HCQ, and chlorpromazine act only by a physical effect, then using them as starting points for more potent drugs could be a waste of time. They present their results as if they prove that this strategy is fatally flawed, but it is not. It is a translational research problem: rather than throwing out all existing drugs, we need to find out what is needed to get the demonstrated results in cells to happen in people.
One commentator recommends computer modeling as an adjunct to drug discovery. Computer modeling and other structural biology techniques are valuable to generate ideas and test hypotheses, but they're not a magical infallible way of designing new drugs. Realistically, there is no alternative to understanding the basic biology of this virus. That's important before proceeding to clinical trials. It's also important before you throw out everyone else's babies with your bathwater.
1. WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021 Feb 11;384(6):497–511. doi: 10.1056/NEJMoa2023184. PMID: 33264556; PMCID: PMC7727327.
2. Amani B, Amani B, Zareei S, Zareei M. Efficacy and safety of arbidol (umifenovir) in patients with COVID-19: A systematic review and meta-analysis. Immun Inflamm Dis. 2021 Aug 4. doi: 10.1002/iid3.502. PMID: 34347937.
3. Williams CG, Jureka AS, Silvas JA, Nicolini AM, Chvatal SA, Carlson-Stevermer J, Oki J, Holden K, Basler CF. Inhibitors of VPS34 and fatty-acid metabolism suppress SARS-CoV-2 replication. Cell Rep. 2021 Aug 3;36(5):109479. doi: 10.1016/j.celrep.2021.109479. PMID: 34320401; PMCID: PMC8289695.
4. Tarantino N, Santoro F, Correale M, De Gennaro L, Romano S, Di Biase M, Brunetti ND. Fenofibrate and Dyslipidemia: Still a Place in Therapy? Drugs. 2018 Sep;78(13):1289–1296. doi: 10.1007/s40265-018-0965-8. PMID: 30159817.
5. Yan B, Chu H, Yang D, Sze KH, Lai PM, Yuan S, Shuai H, Wang Y, Kao RY, Chan JF, Yuen KY. Characterization of the Lipidomic Profile of Human Coronavirus-Infected Cells: Implications for Lipid Metabolism Remodeling upon Coronavirus Replication. Viruses. 2019 Jan 16;11(1):73. doi: 10.3390/v11010073. PMID: 30654597; PMCID: PMC6357182.
6. Gowdy KM, Fessler MB. Emerging roles for cholesterol and lipoproteins in lung disease. Pulm Pharmacol Ther. 2013 Aug;26(4):430-7. doi: 10.1016/j.pupt.2012.06.002. PMID: 22706330; PMCID: PMC3466369.
7. Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015 Feb;15(2):104–116. doi: 10.1038/nri3793. PMID: 25614320; PMCID: PMC4669071.
8. Tummino TA, Rezelj VV, Fischer B, Fischer A, O'Meara MJ, Monel B, Vallet T, White KM, Zhang Z, Alon A, Schadt H, O'Donnell HR, Lyu J, Rosales R, McGovern BL, Rathnasinghe R, Jangra S, Schotsaert M, Galarneau JR, Krogan NJ, Urban L, Shokat KM, Kruse AC, García-Sastre A, Schwartz O, Moretti F, Vignuzzi M, Pognan F, Shoichet BK. Drug-induced phospholipidosis confounds drug repurposing for SARS-CoV-2. Science. 2021 Jul 30;373(6554):541–547. doi: 10.1126/science.abi4708. PMID: 34326236.
9. Anderson N, Borlak J. Drug-induced phospholipidosis. FEBS Lett. 2006 Oct 9;580(23):5533–5540. doi: 10.1016/j.febslet.2006.08.061. PMID: 16979167.
10. Pinto D, Sauer MM, Czudnochowski N, Low JS, Tortorici MA, Housley MP, Noack J, Walls AC, Bowen JE, Guarino B, Rosen LE, di Iulio J, Jerak J, Kaiser H, Islam S, Jaconi S, Sprugasci N, Culap K, Abdelnabi R, Foo C, Coelmont L, Bartha I, Bianchi S, Silacci-Fregni C, Bassi J, Marzi R, Vetti E, Cassotta A, Ceschi A, Ferrari P, Cippà PE, Giannini O, Ceruti S, Garzoni C, Riva A, Benigni F, Cameroni E, Piccoli L, Pizzuto MS, Smithey M, Hong D, Telenti A, Lempp FA, Neyts J, Havenar-Daughton C, Lanzavecchia A, Sallusto F, Snell G, Virgin HW, Beltramello M, Corti D, Veesler D. Broad betacoronavirus neutralization by a stem helix-specific human antibody. Science. 2021 Aug 3:eabj3321. doi: 10.1126/science.abj3321. PMID: 34344823.
aug 07 2021, 6:48 am. updated aug 08 2021, 1:01 pm
