 ven if by some miracle Biogen's aducanumab
should be shown to be effective in delaying the progression of Alzheimer's disease,
that doesn't mean we'll have a cure for it. In fact, it's unlikely that we'll ever
find a cure until we understand the pathogenesis of the disease: what causes it
and why the body doesn't fix whatever is going wrong. And that won't happen until
somebody starts funding it.
ven if by some miracle Biogen's aducanumab
should be shown to be effective in delaying the progression of Alzheimer's disease,
that doesn't mean we'll have a cure for it. In fact, it's unlikely that we'll ever
find a cure until we understand the pathogenesis of the disease: what causes it
and why the body doesn't fix whatever is going wrong. And that won't happen until
somebody starts funding it.
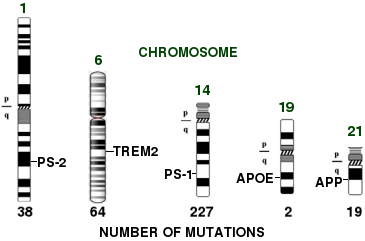
Genes involved in Alzheimer's disease
You might think they're pouring money into this, but they're not: fully half of the National Institutes of Health's current batch of Program Announcements, called PAs, are for clinical trials or clinically-related studies. As far as the NIH is concerned, basic research is no longer wanted: the last exciting research project was on genome-wide association studies or GWASs, which found . . . well, not much besides TREM-2, a gene involved in inflammation. The rest are for things like public health surveys or projects like testing the hundreds of genes that were found in those GWASs to identify their function—a worthwhile, but relatively uninteresting—and unpromising—task of cleaning up the scraps of someone else's project.
There's an oddity here: above is one of my old slides showing the genes that are involved in Alzheimer's. Since this slide was made, there have been a few new ones, but what it shows is that there are 38 different mutations in presenilin-2 (PS-2), 227 different mutations in presenilin-1 (PS-1), 2 in apolipoprotein E (ApoE), and 19 in amyloid precursor protein, or APP, that are strongly associated with AD. If you add in the targets that are weakly associated, which is what GWAS studies tell you, you get dozens, maybe hundreds, more.
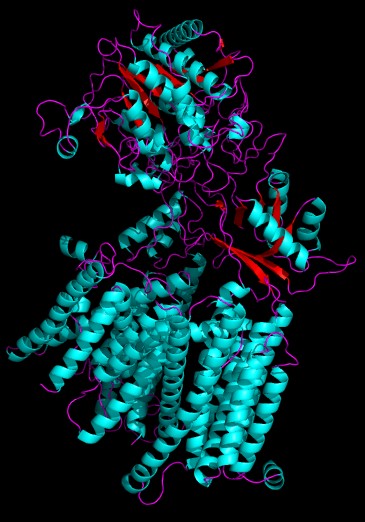
3D Structure of presenilin-1, the catalytic unit of gamma-secretase (cryo-EM, 5FN3) [1] Rendered in pymol
The pharma industry and academia focus on those APP mutations because beta-amyloid, which accumulates in AD, is made by cleavage of APP. Most people still think the goal is to get rid of beta-amyloid, even though no one knows why it's there. Maybe, they're saying, the oligomerized form is toxic, or maybe it binds to tau or ApoE. But fewer than one percent of patients have AD that's caused by mutations in APP. The vast majority of patients with familial or early-onset AD, which is about five percent of all AD patients, have a mutation in presenilin-1, also known as PS-1. And that should be a big clue. Nature is trying to tell us, but we're not listening.
What is gamma-secretase
PS-1 is a component of a huge protein complex known as gamma-secretase. Gamma-secretase is one of two enzymes that cleaves APP to produce beta-amyloid (the other one being beta-secretase or BACE1). But here's the kicker: for years it was thought, or at least assumed, that the reason gamma-secretase was a risk factor was that the mutations were causing it to produce more beta-amyloid, or at least causing it to make more of a different form of beta-amyloid called Aβ42, which tended to aggregate spontaneously compared to the normal form known as Aβ40.
In 2017 a group at Tsinghua University in Beijing dropped an H-bomb on that theory.
In a paper[2] that represents an almost unimaginable amount of tedious work, they cloned 138 different forms of PS-1 known to produce AD in humans and tested their ability to produce beta-amyloid. They found that 90% of the mutations reduced the amount of beta-amyloid. Ten percent of the mutations reduced the ratio of Aβ42 to Aβ40 instead of increasing it as the theory predicted. They wrote:
There is no statistically significant correlation between the Aβ42/Aβ40 ratio produced by a γ-secretase variant containing a specific PS1 mutation and the mean age at onset of patients from whom the mutation was isolated.
This finding has held up, mainly because no one else is ever likely to get enough technical support or funding to clone 138 proteins to determine their function. Considering that there are six forms of gamma-secretase and at least 149 known substrates, and considering that a mutation in PS-1 in an AD patient could cause a shift among any of the six enzyme forms to favor or disfavor any of a hundred different reactions, the task seems astronomical in scope. But what it does mean is that the beta-amyloid theory, which was the driving force in AD research for over thirty years, as currently conceived is dead and buried.
Notch and senescence
Well, as my grandparents would say, that's a fine how do you do. It turns out that gamma-secretase doesn't just cleave APP. As I mentioned, it also cleaves 148 other proteins including LRP1, ERBB4, CD44, Nectin-1α, E-cadherin, and Notch. Many of these are proteins that are involved in maintaining the brain in a healthy state.
Notch is one of the most interesting ones. A mutation in Notch3 causes a fatal neurodegenerative disease called cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, or CADASIL for short. CADASIL became famous when some researchers hypothesized that the philosopher Friedrich Nietzsche suffered from it instead of syphilis as everybody thought. Notch is also essential in the creation of new neurons, known as neurogenesis, and it's involved in cellular senescence.
Cellular senescence has got to be the most complicated biochemical pathways in all of science. I won't make your brain melt down trying to describe it, but essentially when Notch is active you get immunosuppression. When Notch is inactive, you get a proinflammatory, fibrotic state. Fibrosis is relevant because we see lots of collagen in the brains of patients who died of neurodegenerative diseases, though it's impossible to publish that because no one seems to think it's important.
A new theory is needed
Cellular senescence also can occur when your DNA gets damaged or when you get exposed to high doses of radiation. Essentially your cells stop dividing; if they try to divide, a protective mechanism called apoptosis, or programmed cell death, kicks in and kills them to protect the rest of the body.
But there are almost no papers on this: neurons are already unable to divide, so everybody thinks senescence can't be important in AD. We can't afford to study these things. No American in academia could ever test 138 clones just to study Notch. They wouldn't even be able to get it funded, because grant reviewers would say if gamma-secretase has weird effects on APP, it'll have weird effects on Notch, so why bother?
We're like the drunk looking for his keys where the light is better. What's needed is a basic understanding of now the enzyme really works. That's the equivalent of looking in the shadows. Doing it would mean years with no publications and no grants. By the time the researchers finished, their university would have gotten rid of them.
So, how do you do a clinical trial on a drug, which is what the government wants, when you don't know the pathogenesis of the disease and nobody wants to pay to discover it? Easy: you take a wild guess. We've had over 600 of those wild guesses so far. Not a single one has been right. Universities are utterly dependent on government grants brought in by their research faculty. So when the government decides that the way to go is to try something crazy, that's what they do, whether it makes sense to or not.
It's not a failing of any individual, but of the system. Government rewards wild guesses and academia is totally dependent on government. How can science avoid situations like this? That will be the topic of my next article.
1. Bai XC, Rajendra E, Yang G, Shi Y, Scheres SH. (2015). Sampling the conformational space of the catalytic subunit of human gamma-secretase. Elife Dec 1;4. pii: e11182. doi: 10.7554/eLife.11182. PMID: 26623517
2. Sun L, Zhou R, Yang G, Shi Y (2017). Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Abeta42 and Abeta40 peptides by gamma-secretase. Proc Natl Acad Sci U S A. 114(4), E476–E485. doi: 10.1073/pnas.1618657114 PMID: 27930341 PMCID: PMC5278480
jul 03 2020, 5:51 am. last edited jul 07 2020, 9:33 am
