 ast week a group of researchers reported[1] that neurons in Alzheimer
patients produce mutant forms of amyloid precursor protein, or APP. This protein
is important because, when it's chopped up by proteolytic enzymes, you get
β-amyloid, a peptide that has been found in amyloid plaques.
ast week a group of researchers reported[1] that neurons in Alzheimer
patients produce mutant forms of amyloid precursor protein, or APP. This protein
is important because, when it's chopped up by proteolytic enzymes, you get
β-amyloid, a peptide that has been found in amyloid plaques.
The theory that β-amyloid causes Alzheimer's disease, or AD, has been around since the 80s. Mutations in APP can cause early-onset AD, but these represent only a small percentage (<5%) of patients. Without the mutation, AD doesn't occur until age 65 or later, and it's called sporadic or late-onset AD. β-Amyloid is greatly increased in AD patients regardless of whether they have the mutation, and nobody knows for sure why. A somatic mutation would explain a lot.
This paper contains a remarkable amount of data, and probably represents a couple of years of work for the eleven authors. It was accepted in 6 days, which could well be a record for Nature.
What was found?
What they found is that late-onset AD patients have a lot of mutations—6,299 to be exact—in the DNA coding for the APP protein. Eleven of them happen to be mutations found in the rare inherited forms of AD. By contrast, “only” 1,084 forms of APP were found in non-diseased patients. In each case, only 10–12% of these sequences were in frame, meaning only a small minority could ever produce a real protein.
The authors make a big deal about the fact that 11 of the 6,299 mutations were identical to mutations found in early-onset AD, suggesting that the etiology in early-onset and late-onset is the same. However, this is not likely to be meaningful. There are 49 APP mutations that cause AD,[2] so 11 matches could be expected by chance. (There are also 215 mutations in presenilin-1 and 13 in presenilin-2 that cause AD.) Mutations can happen when cells are exposed to ionizing radiation or hydrogen peroxide (H2O2), which is elevated in AD patients. This is called oxidative stress. The authors say reverse transcriptase was converting the mutated RNA back into DNA, potentially making the change permanent in the cell. They had only a small number of patients, but there was a statistically significant correlation between disease and cells with increased gencDNAs, as they call them.
They speculate that the variant sequences could explain why immunotherapy has not worked. Maybe, they say, the antibodies are not recognizing the mutant APP proteins. It is not yet clear what percentage of cells contained mutant APP proteins. To explain the immunotherapy failure, the mutant ones would have to be more abundant or more toxic than the normal ones.
They also speculate that the involvement of reverse transcriptase may explain why AD is rare in AIDS patients who receive anti-retroviral drugs. If so, these drugs could have value in preventing (though not necessarily treating) AD and Down syndrome, in which chromosome 21, which contains APP, is duplicated. More research is needed before this is worth testing, but the authors believe it, and have patented it already. Even more speculatively, they suggest that it could be a natural way of encoding learned information in the genome. This is unlikely considering that 90% of the mutations produced junk instead of a protein.
What do the findings mean?
The presence of multiple forms of APP in the brain has been known for decades. APP, like many big proteins, undergoes genetic rearrangement at the RNA level. It also gets phosphorylated and glycosylated, in which phosphate and sugar molecules are attached. These changes are called maturation and were already known when I ran a similar experiment back in 2002.

In the movies, creating mutations in B-Raf serine/threonine kinase and deleting FOXP2 gives you super strength. (Scene from Hanna)
In my experiment, I found at least 21 forms of APP in Alzheimer's brain. In those days, the best we could do was detect them on 2D gels. There were several different forms in the non-diseased controls as well, but we couldn't determine their protein sequences because we couldn't get enough brain tissue, so we gave up. Lee et al. used sophisticated DNA techniques to get enough sample to sequence—even more remarkably, they used the old Sanger method, where DNA was sequenced using gigantic glass plates and colored dyes. Plates aren't used anymore, but DNA gels are familiar from all those movies about genetically-altered people with super-frizzy hair who discover, to their dismay, that they were born in a lab.
What makes the finding potentially important is the idea that genetic recombination may occur in the brain. Previously, it was only seen in the immune system. The body can make hundreds of billions of different antibodies—far too many to be encoded by DNA. Instead, T cell receptor genes are assembled using a process called V(D)J recombination. There are 44 variable (V) segments, 27 diverse (D) segments, and 6 joining (J) segments that get shuffled around to create many, many new proteins. A similar process happens for immunoglobulins, aka antibodies, in B cells.
These proteins are different types of antibodies. They're called receptors when they're attached to a B cell or T cell, and antibodies when they're floating around in the blood. The antibody parts of the proteins are the same, but the parts that anchor them are different.
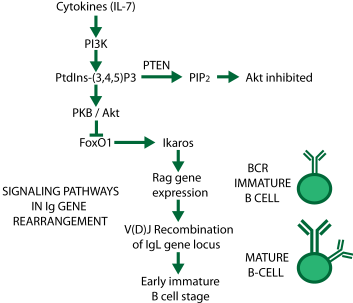
Pathways for genetic rearrangement in the immune system. When the phosphatase PTEN removes a phosphate from PtdIns(3,4,5)P3, FoxO1 is released from inhibition, starting the process.
The process is very complicated, and Tonegawa received the Nobel Prize for unraveling it. It doesn't happen by itself; inflammatory proteins called cytokines trigger a biochemical pathway involving kinases (PI3K and PKB) and phosphatases (PTEN) (see diagram from one of my old slides at right). These enzymes add and remove phosphate groups from other molecules, causing the expression of Rag proteins (= recombination activating genes). Rag protein activates more proteins that chop up certain DNA molecules and reassemble the pieces to make new antibodies. Each B cell thus gets a different antibody, and the antibodies that are needed are selected by fitness, much in the same way as species evolve in nature.
The authors suggest the same thing happens in the brain with APP. If true, it raises two questions: First, what triggers it? If it's cytokines, then AD could be part of an immune response. If it's peroxide or something similar, then we need to take a closer look at where those oxidizers are coming from. Secondly, what do all these different proteins do?
However, there are other possible explanations for the results. For instance, there might be some general deficit in DNA repair. Or maybe DNA translation, which requires energy from mitochondria, is impaired in AD patients. Or it could all just be peroxide chewing up the DNA. Whatever is going on, it breathes new life into the β-amyloid hypothesis—but just barely. It's a finding everybody hoped would be there, but skepticism is definitely in order.
I suspect what it really means is that Alzheimer's is a lot like cancer, where individual cells get a somatic mutation in some protein. In cancer, that mutation causes uncontrolled cell growth. With neurons, that's not possible, and you get neuroinflammation and cell death. If so, we could think of APP as the neuron equivalent of an oncogene.
One thing it means is that we all have to read up on immunology. If it's real, there could be more similarities between the brain and the immune system. Look for hordes of neurologists wandering around in bookstores looking for copies of Janeway's Immunobiology.
[1] Lee MS et al. (2018). Somatic APP gene recombination in Alzheimer's disease and normal neurons. Nature https://doi.org/10.1038/s41586-018-0718-6
[2] Giri M et al. (2016). Genes associated with Alzheimer's disease: an overview and current status. Link Clinical Interventions in Aging 11, 665–681. https://dx.doi.org/10.2147/CIA.S105769
nov 25 2018, 8:45 am. last edited jan 22 2019, 5:11 pm
