Editorial comment While we appreciate the editors of Lancet taking time from their busy schedules to lecture Americans who to vote for, this website has decided that it's more important to examine the pathogenesis of COVID-19. After the pandemic is over, we'll get back to bashing our political opponents, just like The Lancet, we promise!
 any reports have appeared describing symptoms of hyper-inflammation in COVID-19,
including disseminated intravascular coagulation (DIC), neurological disorders such
as acute hemorrhagic necrotizing encephalopathy, and a Kawasaki disease-like syndrome
in young children.
any reports have appeared describing symptoms of hyper-inflammation in COVID-19,
including disseminated intravascular coagulation (DIC), neurological disorders such
as acute hemorrhagic necrotizing encephalopathy, and a Kawasaki disease-like syndrome
in young children.
It's been known from the beginning that cytokine storm is a complication of COVID-19 infection. This is triggered by interleukin-6 (IL-6), which activates what used to be called the acute phase response of the innate immune system, where cells in the liver and elsewhere overreact. But cytokine storm doesn't explain why some patients get an asymptomatic infection and others die.
This article will discuss a new theory that COVID-19 induces a state of partial immunodeficiency that may explain why some patients get cytokine storm and others don't. Before you can understand the theory, there are a few terms that must be explained first.
Acute necrotizing encephalopathy (ANE)
ANE or acute hemorrhagic necrotizing encephalopathy (AHNE) is more of a description of the pathology than a distinct disease. Like acute disseminated encephalomyelitis,[1] AHNE occurs mainly in children after an infection such as influenza, but without direct viral invasion. It differs from ADE in that demyelination isn't observed. It can also be a genetic disorder, possibly of RANBP2.[2] T2 FLAIR MRI shows symmetrical hyperintensities primarily in the thalamus, but also sometimes in brainstem, cerebral white matter, and cerebellum[3] with ring enhancement.[4] Its cause seems to be breakdown of the blood-brain barrier. It is associated with large elevations of IL-6.[5]
Disseminated intravascular coagulation (DIC)
DIC is a complication of septic shock and traumatic injury. In septic shock it's associated with platelet loss (thrombocytopenia) with prolongation of prothrombin time. Sepsis causes hyperventilation and hypoxemia as respiratory muscles become fatigued. Depletion of clotting factors may induce bleeding. D-dimer is a marker.
Kawasaki's disease
A syndrome resembling Kawasaki's disease has been noted in some children exposed to COVID-19. Kawasaki's disease is a necrotizing arteritis characterized by fever, ulceration, nonexudative bilateral conjunctivitis, lymphadenopathy, a polymorphous rash, and ischemic cardiac symptoms. If it spreads to the cerebral arteries, it can cause diffuse encephalopathy or stroke. It is thought to be an immune disorder affecting both the innate and adaptive immune systems. Infectious, environmental, and genetic factors have all been implicated.
Acute phase
Acute phase is a somewhat vague term that refers to release of so-called acute phase proteins called C-reactive protein and serum amyloid A along with ferritin, complement proteins, and liver enzymes. It is caused by self-amplifying release of IL-6 and it's an early predictor of disease severity.[6]
Human cells contain pattern-recognition receptors including TLR-7 and TLR-8, which detect RNA from viruses like SARS-CoV-2. Viral RNA differs from host RNA in that viral RNA isn't end-capped, so TLR-7 and 8 can easily distinguish it from normal mammalian RNA.
The receptors send a signal to the nucleus via the NF-κB pathway.
Virus Receptors
Viruses don't just float through the cell membrane; they must bind to a specific protein, called a receptor. The receptor may import the virus (as in the case of SARS viruses) or merely hold it in place, allowing the RNA or DNA to be injected into the cell. In bacteriophages, which are viruses that infect bacteria, this DNA is contained by a tough protein shell and is packed so tightly that the virus interior is at a pressure of 870 pounds per square inch.[7]
Coronaviruses have large spikes projecting from a lipid envelope that is derived from the cell membrane of the host. The spikes are made of spike protein called S. S protein binds to angiotensin-converting enzyme 2 (ACE2). S protein has two subunits, S1 and S2. The boundary between these two subunits is cut by a protease called TMPRSS2. This cleavage allows the virus to enter the cell.
Interferons
Before getting to immunodeficiency, we also have to know about interferons.
-
Type 1 ("viral"; alpha- and beta-interferon). Induced directly in almost
all cells by virus DNA/RNA acting on Toll-like receptors (TLRs) and RNA helicases. They
promote viral resistance. Mice lacking Type 1 interferons are highly susceptible
to virus infections. Alpha and beta have opposite effects.
- Interferon-alpha has antiviral effects. It is often used as a drug. It has to be injected intravenously.
- Interferon-beta suppresses the immune response. It is sometimes used to treat autoimmune disorders.
- Type 2 ("immune"; gamma-interferon): Made by T cells and NK (natural killer) cells during the immune response. They produce inflammation by signaling the presence of infectious agents to other cells.
- Type 3: Signal through other proteins, ignore for now.
SARS-CoV-2 produces an immunodeficiency-like syndrome
Okay, now for the theory.
We usually think the adaptive immune system, which makes highly specific antibodies and T cell receptors, is irrelevant at this early stage. But SARS-CoV-2 also attacks T cells by binding to a protein on the lymphocytes called CD147.[6] CD147 protects against cancer, especially under conditions of hypoxia. In general, about 85% of critically ill COVID-19 patients have lymphopenia (meaning they have, in general, fewer T cells), but there are suggestions that Treg cells, a type of T cell that regulates inflammation, are especially decreased in COVID-19 patients, while the number of T-helper 17 T cells is increased.[8]
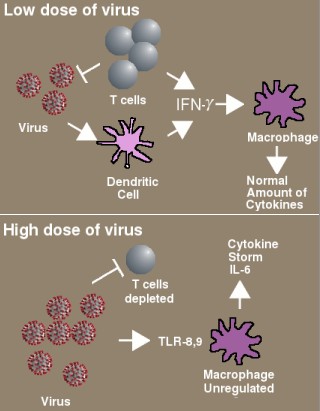
Immunodeficiency in COVID-19 proposed by McGonagle et al. [10]. With a low dose of virus, T cells and dendritic cells produce normal amounts of interferon, which activates macrophages to destroy the remaining virus particles. With a high dose of virus, the T cells are depleted and less interferon is produced. The macrophages over-react, releasing large amounts of IL-6.
SARS-CoV-2 also has a furin cleavage sequence, made of basic amino acids (PRRARS). SARS-CoV-2 is the only SARS virus that has this site. It somehow picked up the four amino acids necessary for cleavage by furin, which is a ubiquitous protease that is also involved in the pathogenesis of anthrax toxin. It is something of a mystery how this furin cleavage site got into the virus. It's also not yet known how the T cells are dying, other than it appears to be due to apoptosis, and it's also not known whether it has anything to do with furin. One suggestion is that the inflammasome may be activated and releases IL-1β which causes pyroptosis, which is a form of programmed cell death like apoptosis.[9]
Cytokine storm, also known as haemophagocytic lymphohistocytosis or HLH, can also occur in genetic disorders in which mutations that impair the function of NK and CD8+ cytotoxic T cells. Like COVID-19 patients, these immunodeficient patients get DIC due to circulating activated macrophages. Their inability to clear pathogens causes the cell to release interferon-gamma, which travels to macrophages and causes massive outpourings of cytokines (including IL-6).
A new paper[10] says the virus is actually causing a form of immunosuppression, whereby the type 1 interferon responses are blocked. It might sound counterintuitive that a disease characterized by hyperinflammation could be a type of immune deficiency, but it fits what we know about the immune system. Here is their theory:
- Normally, interferon-gamma and interferon-beta inhibit replication of SARS-CoV.
- Interferon-gamma signaling in the lung is blocked in COVID-19 (because the viral N and open reading frame (ORF) proteins inhibit it).
- T cells are depleted, which means the type-1 interferon pathway doesn't happen and the infected cells don't get killed.
- Because the virus is now not being cleared by the T cells, more interferon-gamma is released from the macrophages and massive amounts of IL-6 are produced in an attempt to deal with it.
- Patient has a cytokine storm. This (say the authors) explains why most patients get no symptoms and a few get fatal disease—it all depends on how many virus particles they get. Most get a small number; but those who get too many they lose T cells and get cytokine storm as a compensatory effect.
This theory may need tweaking—there are a few pieces still missing—but if true, it could explain why there seem to be two different bifurcated responses to the virus.
Interferon induces the synthesis of ACE2
ACE2 is the receptor for SARS-CoV-2, and binding causes internalization of the virus, so you might think that eliminating ACE2 would prevent infection. This could explain the strange effects of smoking: nicotine decreases ACE2 expression, and so smokers have a much lower incidence of COVID-19. But once heavy smokers get the disease, they are more likely to die from it.
But ACE2 is there for a reason, and some scientists now think it has some protective function against the virus. Indeed, a new paper claims that interferon-alpha-2 drives ACE2 expression.[11] The authors propose that angiotensin II causes lung injury. ACE2 degrades angiotensin II, so binding of the virus depletes ACE2, so angiotensin II is no longer degraded, causing it to rise to toxic levels. ACE2 mRNA is very low abundance, making it hard to measure, so the results need to be replicated.
Animals in which ACE2 is knocked out have worse response to endotoxin-induced ARDS (acute respiratory distress syndrome), which confirms that ACE2 has a beneficial function. Weirdly, though, in vivo administration of interferons doesn't induce ACE2. This research raises the question which nobody seems to be asking: if ACE2 is really the only receptor for SARS viruses, how would an animal with no ACE2 get infected? Well, they probably couldn't, which is why they had to use endotoxins to give them ARDS. It seems that not getting infected in the first place would be better than getting them infected and then curing them. But certainly understanding what ACE2 does is also important.
Update, July 07 2020: One article on this topic, published in Apr 07 Cellular & Molecular Immunology, titled SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion by Xinling Wang et al. has been retracted by the authors. https://www.nature.com/articles/s41423-020-0424-9 https://doi.org/10.1038/s41423-020-0424-9
1. Roos KL, Miravalle A (2014). Postinfectious encephalomyelitis. In Infections of the central nervous system, 4e Chapter 22, pp 331–339
2. Singh RR, Sedani S, Lim M, Wassmer E, Absoud M. (2015). RANBP2 mutation and acute necrotizing encephalopathy: 2 cases and a literature review of the expanding clinico-radiological phenotype. Eur J Paediatr Neurol. 19(2), 106–113. doi: 10.1016/j.ejpn.2014.11.010.
3. Wong AM, Simon EM, Zimmerman RA, Wang HS, Toh CH, Ng SH (2006). Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. AJNR Am J Neuroradiol. 27(9) 1919–1923.
4. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B (2020). COVID-19 associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. Radiology Mar 31. doi: 10.1148/radiol.2020201187. PMID: 32228363 DOI: 10.1148/radiol.2020201187
5. Lin YY, Lee KY, Ro LS, Lo YS, Huang CC, Chang KH (2019). Clinical and cytokine profile of adult acute necrotizing encephalopathy. Biomed J. 42(3), 178–186. doi: 10.1016/j.bj.2019.01.008. PMID: 31466711 PMCID: PMC6717751 DOI: 10.1016/j.bj.2019.01.008 https://www.ncbi.nlm.nih.gov/pubmed/31466711
6. Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, O'Mahony L, Gao Y, Nadeau K, Akdis CA. (2020). Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy May 12. PMID: 32396996 DOI: 10.1111/all.14364 https://www.ncbi.nlm.nih.gov/pubmed/32396996
7. Flint J, Racaniello V, Rall G, Skalka A, Enquist L (2015). Principles of Virology, 4th ed., Vol 1, p.150.
8. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. (2020). Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. Mar 12. pii: ciaa248. doi: 10.1093/cid/ciaa248. PMID: 32161940 PMCID: PMC7108125
9. Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP (2020). The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. Apr 28 doi 10.1038/s41577-020-0311-8. PMID: 32346093 PMCID: PMC7187672
10. McGonagle D, Sharif K, O'Regan A, Bridgewood C (2020). The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease Autoimmun Rev. Jun;19(6), 102537. doi: 10.1016/j.autrev.2020.102537. PMID: 32251717 https://doi.org/10.1016/j.autrev.2020.102537
11. Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH 2nd, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J (2020). SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. pii: S0092-8674(20)30500-6. doi: 10.1016/j.cell.2020.04.035. PMID: 32413319 DOI: 10.1016/j.cell.2020.04.035 https://www.ncbi.nlm.nih.gov/pubmed/32413319
may 17 2020, 11:23 am. edited 3:35 pm. diagram added 4:55 pm
