 ver the past month, we've seen many conflicting reports about the safety and
effectiveness of hydroxychloroquine (HCQ). Now a retrospective study from Tongji
Medical College in Wuhan[1] has the most spectacular result ever.
ver the past month, we've seen many conflicting reports about the safety and
effectiveness of hydroxychloroquine (HCQ). Now a retrospective study from Tongji
Medical College in Wuhan[1] has the most spectacular result ever.
They report that of a total of 568 critically ill patients, all in the same hospital, 48 received hydroxychloroquine, 200 mg 2×/day for 7–10 days, for a total of 4000 mg. Of these 48 HCQ patients, 9 (18.8%) died. Of the 520 non-HCQ patients, 238 (45.8%) died. The difference is highly statistically significant at p=0.0003. See here for my unofficial peer-review.
This is the most positive study so far on HCQ. Even Raoult's original French study (reviewed here), which gave 1.5 times as much HCQ, only showed a significant change when HCQ was given with azithromycin. The current study uses a much lower dose than the widely reported Brazil study where researchers gave 12,000 mg (three times as much) of chloroquine over the same period, pushing the dose into the toxic range. The current study suggests that this strategy is counterproductive.
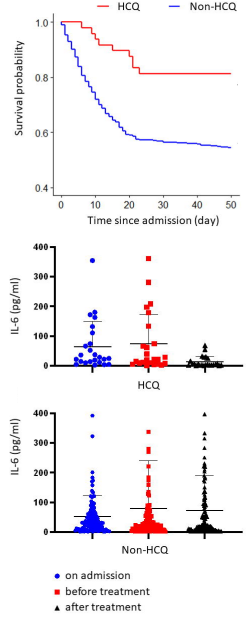
Top: Survival probability graph from Yu et al unpublished article. (note: y-axis starts at 40%, not zero) [1]. Bottom: reduction of interleukin-6
The most interesting finding was that interleukin-6 (IL-6) was reduced from 22.2 (8.3–118.9 pg/ml) to 5.2 (3.0–23.4) pg/ml in the HCQ group but not in the non-HCQ group.
IL-6 is a cytokine that is a key protein in triggering the acute phase response, which is a systemic inflammatory reaction to infection or trauma. The acute phase response induces fever, reduces the number of leukocytes in the blood, and activates the blood coagulation system. We typically measure the acute phase response by monitoring C-reactive protein or serum amyloid A, which can increase up to 1000-fold. If this study is right, it means that HCQ acts by blocking cytokine storm and not by inhibiting virus activation by raising lysosomal pH as previously thought.
These features: blood clots, leukocytopenia, and fever—along with reduced levels of oxygen in the blood, or hypoxemia—are the hallmarks of COVID-19.
C-reactive protein is a pentagon-shaped protein that stimulates clearance of dying cells and danger threats from the bloodstream. It can also be anti-inflammatory by interfering with the interaction of neutrophils with the endothelium. Serum amyloid A is pro-inflammatory and stimulates more cytokine synthesis. It's a key molecule in driving a number of chronic inflammatory conditions like atherosclerosis, amyloidosis, and rheumatoid arthritis. Thus, even a small amount of IL-6 can amplify the production of more, creating a devastating cascade.
This is consistent with several other studies showing that HCQ has little effect on the virus. If the authors of this study are right, it means that the original idea about the mechanism of HCQ was wrong. Inflammation is a tricky thing due to its complexity, and this study suggests that lower doses might work better.
There are certain caveats, namely that it's a retrospective study and not yet officially peer-reviewed. But I could see no glaring flaws in their analysis or statistics. Their subject population is well balanced and their conclusions are reasonable. Only one thing stands out: the death rate among the non-HCQ population was extremely high. These were very seriously ill patients indeed, all of whom were on ventilators, and they were all in CRS (cytokine release syndrome or septic shock). This could explain why no effects were found in other studies.
It may seem counterintuitive that decreasing the dose would improve the results, but I've seen this before in other drugs. Depending on how a drug works, giving too much can trigger compensatory mechanisms that counteract its effect. It demonstrates the necessity of fully understanding the mechanism of action of a drug before running off to a clinical trial. Funding agencies in the USA (not specifying any names here, mind you) should take note.
HCQ is used for systemic lupus erythematosus and rheumatoid arthritis, both of which are autoimmune/autoinflammatory diseases. Its mode of action is thought to be inhibition of Toll-like receptors (TLRs)[2,3], which are proteins involved in the innate inflammatory response. Perhaps other drugs that act on TLR pathways could be beneficial in COVID-19 as well. One example might be Eritoran, which is an antagonist of TLR4 that has been shown to prevent acute lung injury and cytokine storm in influenza.[4]
An ongoing concern with tocilizumab, an antibody against IL-6 receptor, is that suppressing the innate immune system could reduce clearance of the virus. As if on cue, a preprint[5] showed up the very next day showing that HCQ is associated with slower viral clearance. If this is reproducible, it's a big hint that the acute phase response theory may be correct. If so, it suggests that for HCQ, less is more.
1. Yu, B., Wang, D. W., Li, C. (2020). Hydroxychloroquine application is associated with a decreased mortality in critically ill patients with COVID-19 https://www.medrxiv.org/content/10.1101/2020.04.27.20073379v1.full.pdf 10.1101/2020.04.27.20073379
2. Torigoe M, Sakata K, Ishii A, Iwata S, Nakayamada S, Tanaka Y. (2018). Hydroxychloroquine efficiently suppresses inflammatory responses of human class-switched memory B cells via Toll-like receptor 9 inhibition. Clin Immunol. 195, 1–7. doi: 10.1016/j.clim.2018.07.003. https://www.ncbi.nlm.nih.gov/pubmed/29981383 PMID: 29981383 (paywalled).
3. Kyburz D, Brentano F, Gay S (2006). Mode of action of hydroxychloroquine in RA-evidence of an inhibitory effect on toll-like receptor signaling. Nat Clin Pract Rheumatol. 2(9), 458–459. PMID: 16951696 DOI: 10.1038/ncprheum0292 (paywalled).
4. Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, Karp CL, McAlees J, Gioannini TL, Weiss J, Chen WH, Ernst RK, Rossignol DP, Gusovsky F, Blanco JC, Vogel SN. (2013). The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 497(7450), 498–502. doi: 10.1038/nature12118. PMID: 23636320 https://www.ncbi.nlm.nih.gov/pubmed/23636320
5. Mallat J, Hamed F, Balkis M, Mohamed M, Mooty M, Malik A, Nusair A, Bonilla F (2020). Hydroxychloroquine is associated with slower viral clearance in clinical COVID-19 patients with mild to moderate disease: A retrospective study https://www.medrxiv.org/content/10.1101/2020.04.27.20082180v1 doi: https://doi.org/10.1101/2020.04.27.20082180
may 02 2020, 6:43 am. last edited may 03 2020, 5:44 pm
