 n the past decade, aging and diseases of aging such as cancer and Alzheimer's disease
have come to be associated with genetic mosaicism. In this article, I'll describe what
that is and what causes it.
n the past decade, aging and diseases of aging such as cancer and Alzheimer's disease
have come to be associated with genetic mosaicism. In this article, I'll describe what
that is and what causes it.
What is clonal mosaicism
Clonal mosaicism is the presence of different populations of cells in an individual that contain DNA mutations that occur some time during the life of an individual. In some cases the cells contain different karyotypes, meaning a change in the number, size, or shape of the chromosomes in a cell. A mutation can occur in germ cells, making it heritable, but it can also occur in somatic cells, where only one cell has an abnormality. If the cell divides, the daughter cells are also abnormal. If the mutation happens to be one that causes uncontrolled cell growth, these cells can be cancerous.
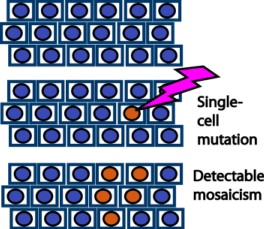
Scheme for clonal mosaicism (redrawn from Machiela and Chanock [ref.2]). If at least 5% of the cells have a specific mutation, it is detectable as a mosaic.
When many different cells in the same organ have different somatic mutations, it is called a mosaic. This can be differences in allele frequency or copy number. Using SNP gene array chips, mosaics can be detected if over 5% of the cells have them. PCR (polymerase chain reaction) or mass spectrometry can also be used. When we search for a specific mutation, it is called genotyping. When we just stain the DNA and look for abnormally-shaped chromosomes under a microscope, it is called karyotyping.
Even with these sensitive techniques, you can only look at a small number of genes at a time. For genotyping, you'd take some cells, then measure the polymorphism using probes. A typical probe has a number like rs429358 or rs7412. Over a million different probes are commercially available.
What causes it
Mosaicing could be caused by an error in DNA repair or by the loss of ability to remove cells with “alternative” genotypes. Mutations can result from smoking, radiation, a cosmic ray, or chemotherapy. Over time, as the cells divide, the mutation is found in a cluster of cells.
This might happen thousands of times in different cells. If the mutation is deleterious, the functionality of the cells declines, leading to chronic disease. Repairing it would mean finding ways to beef up the cell's DNA repair mechanism.
Clonal mosaics in cancer
In 2012, a large group of scientists analyzed DNA from blood samples from 31,818 cancer patients and 26,136 cancer-free controls. They reported[1] that clonal mosaicism increased from 0.23% to 1.91% between the ages of 50 and 75–79. Clonal mosaicism was strongly associated with cancer.
The statistics are impressive: the odds ratio for leukemia was 35.4, meaning that a person's chances of having leukemia were 35.4 times higher if they had this clonal mosaicism. The probability of this happening by chance was about one in 27 billion. In 43 patients for whom the DNA was obtained before cancer was diagnosed, 20% of the ones who later got myeloid leukemia and 22% of the ones who later got lymphocytic leukemia had clonal mosaics, compared to 0.74% in controls.
More importantly, the frequency of mosaic autosomal abnormalities (meaning abnormalities that weren't on the sex chromosomes) increased with age, but the number, size, or proportion of abnormal cells was not correlated with age. The method they used mainly detects mosaic events >2 megabases in size; single-nucleotide mutations need a different method to detect.
Another study, looking at 74 cancer genes, found on average 140 mosaic mutations per square centimeter of skin in normal patients[3]. Even so, we can't say that clonal mosaics automatically cause cancer; there may be other factors that mitigate or enhance their effects. Mosaics could even be part of the cell's normal physiology.
How many mutations are there?
Individual point mutations are rare (1 /megabase), so it's necessary to sequence each gene as many as 500 times to make sure the mutations are real. This is called increased sequencing depth. In cancers induced by mutagens, there are roughly 100 mutations/Mb.[5] In other cancers, such as some bone cancers, pediatric medulloblastoma, and neuroblastomas, cells have chromothripsis or “chromosomal shattering,” which happens when mitosis itself is screwed up (or scrupped as a colleague used to say), and the chromosomes fail to segregate, causing them to undergo aberrant reassembly through a process called nonhomologous end-joining. In these cells there could be hundreds of rearranged genes.
Clonal mosaics in Alzheimer's disease
Some cancer rates are lower in Alzheimer patients, and people have speculated that Alzheimer's is in some ways an “opposite” of cancer, in which cells undergo too much apoptosis (programmed cell death) instead of too little. If true, then treatment would require manipulating the cells' DNA to encourage them to grow. This would mean that finding a cure will be hampered by the difficulty of getting a drug that might cause cancer through the FDA.
A paper in Nature[5] discussed what's happening: by defining a cancer-causing gene as one in which mutations occur more frequently than by chance, we get implausible results, such as that olfactory receptor genes, the cardiac ryanodine receptor, and titin (a huge muscle protein that often shows up in database searches because of its size) are all cancer genes.
The abundance of false positives in cancer research causes problems not just for cancer researchers, but across biology. Recently a dozen or so papers have appeared claiming that a protein called PKCε, a protein that's involved in learning, is an oncogene. This pretty much kills any chance of getting FDA approval for PKCε activators, even though there's strong evidence that the drugs (including some that I designed) are not tumor promoters.
If age-related mutations are important in Alzheimer's disease, it might explain why some patients with inherited mutations get the disease in their 30s, while for most patients, Alzheimer's is associated with aging. Support for this theory comes from the discovery of over 6,000 mutations in neurons of patients dying of late-onset Alzheimer's disease.[6] The authors claimed it was a type of genetic recombination, similar to what occurs in the immune system. But it's more likely to be a form of clonal mosaicism, whereby DNA repair mechanisms are screwed up, allowing DNA mutations to go uncorrected.
Those pesky little DNA molecules are the main problem
DNA gets damaged all the time in cells. Oxidized molecules can cause transcription errors or breaks in the DNA that must be repaired for the cell to continue to express the proteins it needs to survive. Damaged DNA could result from an excess of oxidized molecules or a breakdown in the repair mechanism, which requires energy. This seems to be at least part of what happens in aging.
If so, then the old belief that degenerative diseases such as diabetes, heart disease, cancer, and Alzheimer's are a natural result of aging could turn out to be at least partly true. That will make them tough to cure. But it also means that if a cure is found, it will have benefits far beyond the unfortunate patients who have these diseases. We might not live forever, but at least we'll spend our old age in better health, assuming we don't get hit by a bus first.
1. Jacobs KB et al. (2012). Detectable clonal mosaicism and its relationship to aging and cancer. Nat Genet 44(6), 651–658. Link Free article on PMC
2. Machiela MJ, Chanock SJ The Ageing Genome, Clonal Mosaicism and Chronic Disease Curr Opin Genet Dev. 2017 Feb; 42: 813. Link
3. Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, Wedge DC, Fullam A, Alexandrov LB, Tubio JM, et al. (2015). High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886.
4. Lawrence MS et al. (2014). Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218.
5. Dumanski JP, Lambert JC, Rasi C, Giedraitis V, Davies H, Grenier-Boley B, Lindgren CM, Campion D, Dufouil C, et al. (2016). European Alzheimer's Disease Initiative I. Mosaic Loss of Chromosome Y in Blood Is Associated with Alzheimer Disease. Am J Hum Genet. 98, 1208–1219.
6. Lee MS et al. (2018). Somatic APP gene recombination in Alzheimer's disease and normal neurons. Nature https://doi.org/10.1038/s41586-018-0718-6
jan 11 2019, 7:20 am. edited jan 12 2019, 5:51 am
