 t's no great mystery why so many scientific papers are getting retracted
these days. When scientists say they're in a rat race they don't mean those
cute little white rats. They mean it's a race between the researchers
and those who make their lives difficult.
t's no great mystery why so many scientific papers are getting retracted
these days. When scientists say they're in a rat race they don't mean those
cute little white rats. They mean it's a race between the researchers
and those who make their lives difficult.
This time a study of beta-amyloid in Alzheimer's disease has fallen under suspicion of image manipulation. The finding in question is the existence of an oligomerized form of beta-amyloid called Aβ*56. Once again, it is Western blots that are to blame.
For the record, I never saw Aβ*56 in my samples, though I never explicitly looked for it. I thought the Aβ*56 story was just hype. Maybe it was a transient form, or maybe something that shows up only under certain conditions—in other words, an artifact. But it might be real. Beta-amyloid tends to clump up, and maybe a 12-mer could happen.
The problem was that somebody in their lab supposedly manipulated their Western blot image. That's the problem with Western blots: they're easy to do but they rarely come out perfect, and publications demand perfection or they'll reject your paper.
What is a Western blot, and why do scientists hate them?
Western blots, for those unfamiliar with them, are a way of measuring the amount of a protein in a sample. We apply the proteins onto a polyacrylamide gel—a thin slab of a slimy, transparent, toxic Jell-O-like substance—and separate them in an electric field. Then we transfer them to a membrane using an electric field perpendicular to the gel.
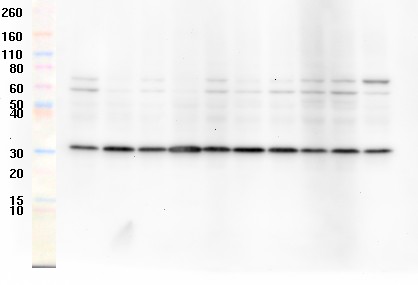
A Western blot. The colored bands on the left are molecular size markers that we now have to include in every image. My blots always come out like this in the lab. Almost always. Sometimes.
Then we dump an antibody on the membrane, wash it off, dump in a second antibody, wash that off, and dump more chemicals on that to make the protein visible. As you might guess, there are many steps involved and each step is where something can go wrong: the protein of interest could get degraded, or the blotting process, which is highly sensitive to variables like pH, can be screwed up. Or you can get artifacts in the blot caused by a bubble or a defect in the membrane that produces an artifact where the protein should be.
Or the antibody may not be as specific as the manufacturer claims. I know a guy who lost months of effort because of that. The company insisted that the antibody was perfect, but he finally discovered they were wrong. Academic researchers are pushed to write papers and grants, so they almost never validate their antibody and must trust the manufacturer.
Then there's the quantitation, which is rarely perfectly linear. Maybe the blot has an uneven background that needs to be corrected. Or maybe a smudge. Or maybe one of a hundred other things went wrong.
And that's just the start. Twenty years ago, researchers made their own gels. Running a blot took three days. When precast gels became available, for a while we could do a blot in one day. Then the demands for more rigor started. The blots had to look perfect. We had to strip the blots and re-stain them with a different antibody to confirm that the protein load really was the same. Every blot had to have visible molecular weight markers on it to prove it was the size we said it was.

This is how academic researchers conserve grant money (custom designed test tube rotator)
Then we needed to use very expensive imagers that can detect infrared light or tiny amounts of luminescence instead of color. But we also have to take color pictures of the gel, because those luminescent markers we get from big name companies quite often show no signal at all. The same with the commercial stripping reagents, which never do what the company claims.
Now it takes a week to run an experiment. We tediously repeat our experiments over and over, hoping the blot will come out publishable.
It's no wonder some people use Photoshop to splice together two or more blots. There's always one sample that doesn't come out right. So instead of taking another month to get a good one, they “fix it in post.” Hey, they're probably thinking, movie producers do it, why not us?
People problems
Often it's a grad student or postdoc who feels under pressure to get reliable results from an unreliable method. That means it's a people problem all the way up to the top, including the PI, the scientific director, the journal editors, and the reviewers who trash manuscripts for having an “unconvincing“ blot.
Many of my collaborators now refuse to run blots at all. They prefer to use ELISA, despite its enormous cost, because publishers will accept an ELISA result without all the extra controls, which are impossible to do on an ELISA.
The problem, then, is not that the results of these retracted papers are necessarily false—they might be, or they could be perfectly valid—but that the researchers yielded to the pressure to get a perfect result with a method that sucks on ice. The researchers will lose their jobs because scientists are supposed to be 100% honest, and rightly so.
What is the solution?
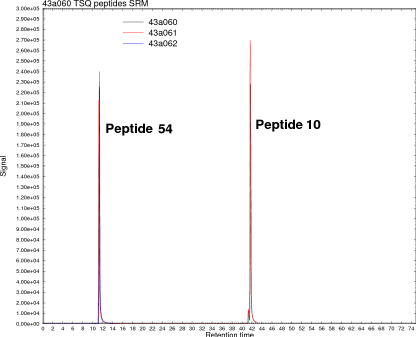
Protein quantitation using mass spectrometry
The peak in the image at right is a protein called PKC epsilon analyzed on a triple-quadrupole mass spectrometer using a method called SRM. It's just as sensitive as an ELISA and maybe a hundred times more specific than an ELISA or Western blot. It's highly reproducible and linear over a wide range. Even better, no antibodies are needed, only the peptide sequence. You digest your protein, buy a peptide (which is cheaper than an antibody), and set your instrument to the correct fragmentation pattern. The identification here was 100% specific, something we could never get on a Western.
There are only a few problems: these machines take up half a room, they're finicky as hell, they cost half a million dollars, and they require a highly experienced person to run and maintain. If a plug-in card goes bad, it can cost $35,000. It takes over thirty minutes to run a sample. Oh, and no detergents or salt can ever be in there.
Despite those drawbacks, something like this is how we'll analyze proteins ten years from now, unless the rule-makers hit us again demanding more and more controls. I'm not sure what they'll demand, but I do know it will make the mass spec method so tedious that in fifteen years we'll be seeing more papers retracted for falsifying results from it. But at least we'll have a good five years of being productive. And maybe some of us can retire and get out of this ridiculous rat race by then.
jul 24 2022, 2:50 pm
