 here is widespread confusion on the Internet about whether COVID-19 is surging.
Some people are saying that up to 2/3 of COVID-19 diagnoses may be false positives.
Others claim the tests are 98% accurate. An example of the confusion was seen when
it was reported that Elon Musk had two positive and two negative test results. This
seemingly impossible report can be explained if we understand what the tests can
and cannot measure.
here is widespread confusion on the Internet about whether COVID-19 is surging.
Some people are saying that up to 2/3 of COVID-19 diagnoses may be false positives.
Others claim the tests are 98% accurate. An example of the confusion was seen when
it was reported that Elon Musk had two positive and two negative test results. This
seemingly impossible report can be explained if we understand what the tests can
and cannot measure.

ELISA plates
How do COVID-19 tests work?
There are three fundamentally different tests in use: RT-PCR tests, which measure the presence of viral RNA, ELISA tests, which can measure virus proteins or human antibodies against viruses, and clinical tests such as CT scans. Only clinical tests actually tell us whether the patient has the disease.
RT-PCR
In RT-PCR (reverse transcriptase polymerase chain reaction or real-time polymerase chain reaction), a sample of the patient's mucus or epithelial cells is sent to a testing laboratory. There the sample is mixed with enzymes called reverse transcriptases that make a DNA copy of the RNA. DNA primers, which are short pieces of DNA complementary to the DNA in the virus, are added to the sample. Here is a typical primer pair used to detect SARS-CoV-2:
CCC TGT GGG TTT TAC ACT TAA (sense)
ACG ATT GTG CAT CAG CTG A (antisense)
In order for the probes to work, one probe must anneal (bind) to one strand of the template and the other must anneal to the other strand of the same template a specific distance away. They could not amplify if, for example, one annealed to DNA from a virus and the other annealed to DNA from a different virus or a bacterial DNA. If the annealing positions are on the same template but not the correct distance apart, a product will be produced but it will have the wrong size, which means the primers are no good.
This means even a 100% match of one primer is not enough. Both primers must match the template and they must be the correct distance apart. The match need not be 100%, but even a single mismatch reduces the efficiency of amplification.
The DNA is then heated to convert it to single-stranded DNA and cooled. If a virus sequence is present, the primers adhere to the virus DNA to form a short stretch of double helix. A small amount of fluorescent nucleotide is present, along with an enzyme called Taq polymerase. The Taq polymerase fills in the missing bases between the sense and antisense primers. At the end of each cycle the machine heats up the DNA again, separating all the strands, and each strand becomes a new template. As the DNA is amplified in the PCR machine, the DNA molecules become fluorescently labeled and the amount of fluorescence as a function of time is plotted on a computer. At the end of each cycle, assuming the reagents aren't depleted, we have:
- The two original long DNA strands (the original templates)
- Two new medium length DNA strands are created in each cycle, caused by one DNA strand being translated all the way from the antisense primer to the start of the template and the other strand translated from the sense primer to the end of the template.
- New short length DNA fragments of the region between the two primers. These fragments are created exponentially with the number of PCR amplification cycles.
The number of short length DNA fragments increases as 2 × the set of Eulerian numbers { 0, 1, 4, 11, 26, 57, 120, 247, ... }. This is known as Euler's triangle series (see pcr-products.png). The Euler's triangle numbers are nothing more than 2 × (2cycles− the number of cycles − 1). For example:
| Cycle no. | 2cycle | No. of short DNA fragments | Amplification factor |
|---|---|---|---|
| 1 | 2 | 0 | 0 |
| 2 | 4 | 2 | 1 |
| 3 | 8 | 8 | 4 |
| 4 | 16 | 22 | 11 |
| 5 | 32 | 52 | 26 |
| 6 | 64 | 114 | 57 |
| 7 | 128 | 240 | 120 |
| 8 | 256 | 494 | 247 |
| 9 | 512 | 1004 | 502 |
| 10 | 1024 | 2026 | 1013 |
| 20 | 1,048,576 | 2,097,110 | 1,048,555 |
| 30 | 1,073,741,824 | 2,147,483,586 | 1,073,741,793 |
| 40 | 1,099,511,627,776 | 2,199,023,255,470 | 1,099,511,627,735 |
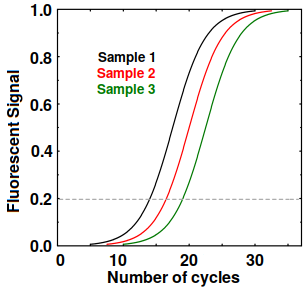
Sigmoidal curves from RT-PCR
Eventually, the reagents do get depleted, so the amplification stops and we get sigmoidal curves as shown at right. If all goes well, all the curves have an identical shape and the curve for each sample can be described by a Ct (cycle threshold) value, which is defined as the number of cycles at which the fluorescent signal minus the background exceeds some value, such as 0.2. This tells us the amount of template in the original sample. Any Ct over 30 or 40 suggests possible contamination or bad primers.
Limitations of PCR
PCR is extremely sensitive. Some test kits can detect one virus particle in every 50 μl of sample. If the virus is a 100 nanometer diameter sphere, this means PCR can detect the equivalent of 1 drop of water in 1000 Olympic-size swimming pools.
Because of its high sensitivity, any contamination from dust, contaminated reagents, or from the technician can give a false positive result. Non-specific amplification, caused by primers binding to DNA other than the desired template, can lead to a false signal. At least 2 sets of primers are needed to reduce false positives. Thus, primers have to be carefully designed to avoid overlap with DNA from bacteria, human cells, or other viruses.
What does a positive PCR test tell you?
Although free RNA is rapidly destroyed, RNA inside a virus particle is protected until it reaches the testing lab. If the test is carried out properly, a positive PCR test will tell you that you were exposed to the virus, which is to say it found one or more virus particles in your sample. This does not necessarily mean you are infected. A low Ct is good evidence that you may be producing viruses, but a high Ct value can be inconclusive. There have been some cases where patients were clinically ill but had high Ct values over 35 or even 40.
In most cases it is the definition of ‘positive’ and the perception of risk, not the PCR technology itself, that people are arguing about.
ELISA tests
An ELISA (enzyme-linked immunosorbent assay) typically uses a small 96- or 384-well plastic plate coated with an antibody that detects the presence of some protein, called an antigen, in the sample. A second antibody labeled with something that allows detection, such as horseradish peroxidase, is added and the signal is measured at a single time point. In the olden days, like four or five years ago, this signal was a color change. Nowadays chemiluminescence is typically used.
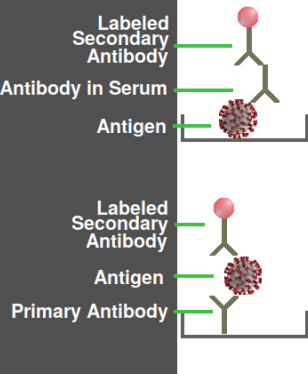
Two variations of an ELISA assay
The antigen need not be an intact virus; most likely it is a sample of the recombinant protein or synthetic peptide that was used to produce the antibody. Thus, there is no danger of viruses escaping from a testing laboratory. The figure at right shows how, depending on how the plate is coated, ELISA could detect either a virus protein or a specific antibody in the patient's blood serum. The latter type is more commonly used in COVID testing.
Advantages and Disadvantages of ELISA tests
The advantage of an ELISA test is that a positive result is evidence (but not proof) that the patient has an immune response to the virus, or something closely resembling the virus. ELISA tests are very easy to carry out: I have even seen medical doctors who were able to do an ELISA.
The biggest disadvantage of ELISA is that antibodies are not always present, even in an infected patient. IgM is only produced between day 7 and 21 of an infection, while IgG is not produced in significant amounts for 1–2 weeks, by which time the patient has hopefully recovered.
Another disadvantage of an ELISA is that ELISA plates are too time-consuming for testing labs to make. Plates are therefore made by big companies, sometimes in foreign countries, which rarely provide materials that would allow testing labs to validate the assay. Thus, labs must rely on the claims of the manufacturer that the ELISA is specific.
Other tests
Because RT-PCR machines cost over $30,000 apiece, many companies have devised simpler tests that could be done in the field. Although many of these tests have been approved by the FDA, they are useful only for screening and need to be followed up by a better test.
LAMP (loop-mediated isothermal amplification). LAMP is similar to RT-PCR except that it is carried out at a constant temperature. Unlike RT-PCR, it does not require a sophisticated computer control. It can be done in any lab and the results can be detected simply by looking at the test tube.
LFIA (lateral flow immunoassays). These are field tests that are easy for untrained people to do. They consist of a paper or microstructured polymer material coated with antibodies and certain chemicals. The patient's blood is dropped on it and bands appear like in a pregnancy test.
Immuno-PCR This technique is like an ELISA, where the protein binds to a plate. As in ELISA, a second antibody is added, except the second antibody is attached to a piece of DNA which is then amplified by PCR. I won't say this is a stupid idea; let's just say there is not yet a viable niche for this test.
Other tests Many kinds of biosensors have been proposed to detect the virus, including nanowire biosensors, surface plasmon resonance sensors (which measure binding of some protein directly), and aptamer tests. CRISPR-Cas-based assays such as SHERLOCK, DETECTR, FELUDA, and CARMEN-Cas13 use novel reagents such as mutated Cas13.
CT (computed tomography). This clinical test shows the presence of a lung infection, i.e. a pneumonia, and observation of ground-glass opacification in the image can suggest COVID-19. This is the only test that definitively indicates that the patient actually has a lung infection.
Mass spectrometry (MS) can detect viruses or antibodies with sensitivity equal to that of ELISA. The advantage is that an extremely high specificity can be obtained. However, mass spectrometers are very expensive and require constant maintenance. If a circuit board on a mass spec goes bad, it can cost more than an entire PCR machine. Therefore, mass spectrometry is still considered a research tool.
Perhaps the biggest obstacle is the strong incentive in overstating the accuracy and sensitivity of the test. This happens because there is pressure to find something that can help patients, but it can also lead to wishful thinking. Publication bias, in which only experiments that appear to work can get published, could lead to widespread skepticism about all tests. Most harmful of all is censorship in social media, in which negative comments are censored while positive stories are allowed to spread. These factors will undoubtedly continue to create doubts and skepticism about COVID testing.
Update, Nov. 27 2020: A September 2020 paper in Clinical Infectious Diseases [1] by several authors including the legendary virologist Didier Raoult and B. La Scola of the IHU Méditerranée Infection institute provides hard data that challenges the connection between RT-PCR positivity and COVID-19 infection. Their clinical lab ran over 250,000 RT-PCR tests on 179,151 patients. They report that at Ct = 25, about 60–70% of patients remain positive in culture, while at Ct = 35, fewer than 3% of cultures are positive. Their graph shows that the cutoff is at Ct = 26, above which more tests are incorrect than are correct. At cycle 30, fewer than 20% of PCR positives indicate an actual positive viral culture.
1. Jaafar R, Aherfi S, Wurtz N, Grimaldier C, Hoang VT, Colson P, Raoult D, La Scola B. Correlation between 3790 qPCR positives samples and positive cell cultures including 1941 SARS-CoV-2 isolates. Clin Infect Dis. 2020 Sep 28:ciaa1491. doi: 10.1093/cid/ciaa1491. PMID: 32986798; PMCID: PMC7543373. full-text link
nov 17 2020, 6:33 am. edited for brevity nov 17 2020, 6:19 pm. additional paragraphs on primer specificity added nov 22, 2020, 3:43 am. updated nov 27 2020, 4:14 am
