 esterday somebody in my lab submitted a grant proposing that some
protein was involved in a neurodegenerative disease. After struggling
with bad WiFi and power outages that happen in our building every time
it rains or snows or somebody sneezes, they made it twelve minutes before
the deadline.
esterday somebody in my lab submitted a grant proposing that some
protein was involved in a neurodegenerative disease. After struggling
with bad WiFi and power outages that happen in our building every time
it rains or snows or somebody sneezes, they made it twelve minutes before
the deadline.
It's a good proposal and it'll probably get funded. But maybe it shouldn't be.
Suppose you find a change in the amounts of your favorite protein. Let's call it Protein X. In science a rule of thumb is that the size of the change tells you how important the change is to what you're studying. If it changes ten fold, it's highly relevant. If it only changes by 25 percent, not so much.
There are now hundreds of Protein X's and the literature is stuffed with papers describing useless 25% changes. To get them published, authors fluff them up to make the change sound important, but it's no more relevant to the disease than Kim Kardashian's backside is to the national debt.
Our reliance on the bad molecule theory of disease short-circuits our thinking, and it permeates all biomedical research. At least Kim Kardashian's rear end is useful as a metaphor.
We inherited this paradigm from medicine, which bequeathed to us a zoo of bacteria and viruses, which are basically big bundles of bad proteins. The paradigm spread to all of biomedicine like a disease. The results were predictable: big pharma spent billions finding ways to get rid of beta-amyloid without spending a dime trying to figure out why it's there in the first place.

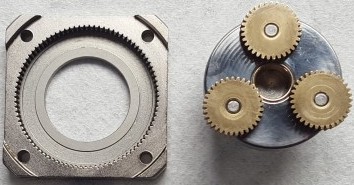
HPLC injector in autosampler. The tangle of black wires and orange wire nuts goes to a nearly inaccessible gear motor which controls the injector valve attached to the brown tubing in the center
Cured!
In general, the difference between medicine and biomedicine / biology is that medical studies look for correlations and biological studies look for mechanisms. A correlation is just that: an observation of a disorder or an effect of a treatment. A mechanism is an explanation of an effect in cellular or molecular terms relating the effect to its ultimate cause.
Finding an effect obviously must come first. But because of the pressure to publish, often that's as far as a study ever goes.
Indeed, correlations are the main product in ‘omics’ studies, where we look at a huge collection of molecules and try to deduce which molecule or which pathway is broken. Omics studies tend to run in fads. We had fads for DNA microarrays, then protein microarrays, then systems biology, now RNASeq. They all followed the same trajectory: people write thousands of papers on it, creating reams of colorful but mostly uninterpretable computer diagrams. Eventually it dawns on everyone that the whole thing is meaningless unless the results point to a mechanism and somebody trusts the omics results enough to spend time proving them. This happens so infrequently that the NIH had to publish an RFA trying to encourage it. We need a new paradigm. Or maybe a pair of paradigms.
Here's a clue. Last week, I was repairing a little gear motor on our HPLC autosampler. It's a small stepper motor hidden behind the steel valve in the center of the photo at right. After fixing all the broken pieces, and finally discovering the magical incantation (which nobody bothered to put in the manual) that the crap software needed before it would work, I finally got it fixed.
This actually tells us a lot about diseases. The clunking and grinding sound made by the motor is not the disease. The millimeter-deep gouge in the stator is also not the disease. The disease is whatever exogenous material got into the valve and seized it up. The symptom here, as in many human diseases, is ‘high blood pressure’ or in this case high solvent pressure.
I've sat helplessly on grant committees where even the most feeble attempts to break out of this paradigm just get trashed—triaged as they call it—while the most idiotic proposals that support the paradigm sail through.
It's not just in biology. At one point tinnitus researchers all hopped onto a fad of their own, using trans-cranial stimulation on the theory that tinnitus was caused by bad neuronal connections in the brain. They eventually discovered that it didn't help.
We measure the amounts of our Protein X and discard the result if there's not a big change, ignoring the fact that proteins don't just exist: they translocate, get post-translationally modified, and they get misfolded and degraded. DNA gets broken or mutated. When harmful pathways like oxidative stress or senescence get activated, treating the oxidative stress or killing the senescent cells isn't treating the disease any more than finding a way to reduce the pressure overload in that pump is treating the ‘disease’ in the HPLC.
There's no such thing as a bad protein or a bad cell. Every molecule in the cell is there for a reason. Unless you know what that reason is and what caused the molecule to stop doing its job, you don't fully understand what's going on. Granted, finding an effect may give us an important clue, but the broken watch metaphor fits: we would never publish a paper saying that a watch gets broken without identifying whether it was the spring, the hands, or the crystal that was broken, and why. Why is it acceptable to do the same with our experiments?
jul 09 2022, 6:35 am. updated jul 10 2022 5:44 am
