|
 his useful but expensive CRC Press book describes the
chemical reactions for chemically synthesizing peptides, a topic that is
constantly changing due to the enormous economic incentives in industry
for improving efficiency and purity. Although it is now possible to buy
custom peptides at a reasonable price, peptide vendors are
frequently unable to synthesize anything other than simple linear peptides.
This means that chemists must occasionally still
know how to make peptides on their own. The reactions themselves are
extraordinarily efficient; unlike the usual 70-80% yields
in ordinary organic chemistry, reactions used for peptide synthesis are
often over 99% efficient. This makes them useful for many other applications
besides making peptides. However, there are very few up-to-date books that
describe these reactions.
his useful but expensive CRC Press book describes the
chemical reactions for chemically synthesizing peptides, a topic that is
constantly changing due to the enormous economic incentives in industry
for improving efficiency and purity. Although it is now possible to buy
custom peptides at a reasonable price, peptide vendors are
frequently unable to synthesize anything other than simple linear peptides.
This means that chemists must occasionally still
know how to make peptides on their own. The reactions themselves are
extraordinarily efficient; unlike the usual 70-80% yields
in ordinary organic chemistry, reactions used for peptide synthesis are
often over 99% efficient. This makes them useful for many other applications
besides making peptides. However, there are very few up-to-date books that
describe these reactions.
Chemistry of Peptide Synthesis is a bona fide textbook, not a collection of articles by different authors. It is clear that much effort has been expended in making the writing style as clear as possible. In fact, the author seems to have written the book as much from strongly-held, almost curmudgeonly opinions about peptide nomenclature as from an interest in the topic itself. For example, as the back cover says, the book "employs only the precise terms of enantiomerization and epimerization." The author emphasizes in no uncertain terms that these two words are quite distinct in meaning. Furthermore, the protecting group Boc, says the author, should never be referred to as "t-Boc", because the 't' is implied by the chemical structure.
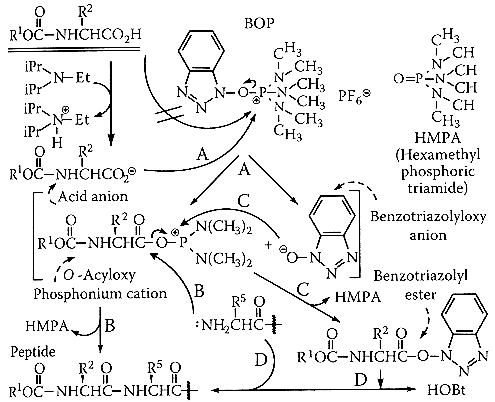
However, the utility of the book is greatly diminished by two major problems. The first problem is the figures. Although the diagrams are readable, or at least decipherable, with some effort, the molecules are all packed together confusingly. Chemical structures are drawn in an unconventional and bulky way. Arrows sometimes indicate the flow of electrons, and sometimes indicate the reaction pathway. Other times, arrows travel long distances to reach other molecules hiding coyly on the other side of the diagram. Arrows appear to come from or go to nowhere, point to other arrows or to chemically unrelated and impossible products, or travel from the name of the molecule to the molecule itself, while other molecules sit lonely by themselves, with no arrows at all (see example below).
The other problem is that the index is organized into grand topics like
"Reagents" and "Names of scientists", in a manner reminiscent of the old
Allied Electronics Catalog, rendering the index almost useless
and making things very difficult to find.
Other than that, the book is very useful. Alternative methods and
reagents are described, along with their benefits and liabilities,
and mechanisms are explained in detail.
The references (mostly to classic papers) are conveniently included
at the end of each section in each chapter.
Unlike Solid Phase Peptide Synthesis: a Practical Approach
by Atherton and Sheppard, no specific procedures are described; the
reader must consult the primary literature to learn the reaction
conditions. However, Solid Phase Peptide Synthesis is now
out of print. Fmoc Solid Phase Peptide Synthesis: A Practical
Approach by Chan and White is by far the best book on the topic,
providing a clear exposition, with readable diagrams, detailed
protocols, and is up to date, making it an ideal choice for those
interested in knowing the standard way of making peptides today.
Unlike many other books, Fmoc Solid Phase Peptide Synthesis
also covers topics like branched peptides.