Biophysics Books
Protein Folding Kinetics: Biophysical Methods, 2nd ed.
Bengt Nölting
Springer, 2006
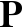 rotein folding is one of the great unsolved problems in biochemistry.
This short textbook first reviews the protein structures that affect
folding, then covers the thermodynamics and reaction kinetics. These
are all topics familiar to most biochemists and enzymologists. After this,
the book describes instrumentation useful in studying ultrafast denaturation
and renaturation kinetics, including temperature jumps, protein engineering,
fluorescence, and time-resolved circular dichroism and NMR.
The remaining chapters discuss experimental problems such as aggregation and
low protein expression levels, and specific examples using barstar, which
is a 10 kDa ribonuclease inhibitor. Computer modeling and software are barely
discussed; the software that is used (Molscript, etc.) is mostly old-fashioned,
but still useful. Most of the references are from 1991 to 1996. The last
chapter briefly describes the author's evolution folding simulation software.
Although the long strings of references in "Name et al., year" format
scattered on almost every page almost kill this book's readability, it
is still a useful introduction to the methods of studying folding kinetics.
rotein folding is one of the great unsolved problems in biochemistry.
This short textbook first reviews the protein structures that affect
folding, then covers the thermodynamics and reaction kinetics. These
are all topics familiar to most biochemists and enzymologists. After this,
the book describes instrumentation useful in studying ultrafast denaturation
and renaturation kinetics, including temperature jumps, protein engineering,
fluorescence, and time-resolved circular dichroism and NMR.
The remaining chapters discuss experimental problems such as aggregation and
low protein expression levels, and specific examples using barstar, which
is a 10 kDa ribonuclease inhibitor. Computer modeling and software are barely
discussed; the software that is used (Molscript, etc.) is mostly old-fashioned,
but still useful. Most of the references are from 1991 to 1996. The last
chapter briefly describes the author's evolution folding simulation software.
Although the long strings of references in "Name et al., year" format
scattered on almost every page almost kill this book's readability, it
is still a useful introduction to the methods of studying folding kinetics.
jan 21, 2012
Methods in Modern Biophysics, 3rd ed.
Bengt Nölting
Dutton, 2004
 his introductory textbook surveys some of the methods used in
biophysics, the study of the physical properties of biomolecules.
Chapters include mass spectrometry, X-ray crystallography, electron
microscopy, de novo peptide sequencing, protein infrared spectroscopy, and
a few other well-established techniques. A few relatively new techniques,
including ion mobility spectrometry and nanotechnology, are also included.
The coverage is light and relatively superficial, with some chapters
only a few pages long. The author is a non-native English speaker, with
all that that implies. One annoyance is that references are in "Name et
al., year" format; many paragraphs are little more than a long string of
references, making the book unpleasant to read. This book might be useful
for non-specialists interested in an overview of the field, but it would be
of very limited value for grad students or professional biophysicists
seeking in-depth understanding.
his introductory textbook surveys some of the methods used in
biophysics, the study of the physical properties of biomolecules.
Chapters include mass spectrometry, X-ray crystallography, electron
microscopy, de novo peptide sequencing, protein infrared spectroscopy, and
a few other well-established techniques. A few relatively new techniques,
including ion mobility spectrometry and nanotechnology, are also included.
The coverage is light and relatively superficial, with some chapters
only a few pages long. The author is a non-native English speaker, with
all that that implies. One annoyance is that references are in "Name et
al., year" format; many paragraphs are little more than a long string of
references, making the book unpleasant to read. This book might be useful
for non-specialists interested in an overview of the field, but it would be
of very limited value for grad students or professional biophysicists
seeking in-depth understanding.
jan 18, 2010
Differential Scanning Calorimetry, 2nd ed.
Höhne, Hemminger, and Flammersheim
Springer, 2010
 his textbook describes differential scanning calorimetry
as used in industry to study inorganics and pure materials.
The authors thoroughly describe instrument calibration,
desmearing, and performance evaluation. There is also a chapter
on using a DSC to measure reaction kinetics.
The treatment leans toward the abstract, with little attention
to data interpretation or the physical and chemical processes in
the sample.
There is no discussion of the use of DSC to study biomolecules,
or of the problems with aqueous samples.
The statement on the back
cover that this book "enables researchers to select the best instrument"
is untrue: the only mention of a physical device is an occasional vague
reference to Perkin-Elmer, which is only one of many manufacturers.
The authors are coy about what DSC is actually good for:
it is not until page 238 that
the authors finally get around to saying what information is
obtained from temperature-modulated DSC (TMDSC).
For those interested in the basic theory, the exposition is clear and
easy to follow. Students looking for a practical introduction
on how to run samples and interpret the results, readers interested in
building or purchasing a machine, and researchers interested
in biomolecules should look elsewhere ... like, maybe, the manual
that came with their machine.
his textbook describes differential scanning calorimetry
as used in industry to study inorganics and pure materials.
The authors thoroughly describe instrument calibration,
desmearing, and performance evaluation. There is also a chapter
on using a DSC to measure reaction kinetics.
The treatment leans toward the abstract, with little attention
to data interpretation or the physical and chemical processes in
the sample.
There is no discussion of the use of DSC to study biomolecules,
or of the problems with aqueous samples.
The statement on the back
cover that this book "enables researchers to select the best instrument"
is untrue: the only mention of a physical device is an occasional vague
reference to Perkin-Elmer, which is only one of many manufacturers.
The authors are coy about what DSC is actually good for:
it is not until page 238 that
the authors finally get around to saying what information is
obtained from temperature-modulated DSC (TMDSC).
For those interested in the basic theory, the exposition is clear and
easy to follow. Students looking for a practical introduction
on how to run samples and interpret the results, readers interested in
building or purchasing a machine, and researchers interested
in biomolecules should look elsewhere ... like, maybe, the manual
that came with their machine.
aug 1, 2010
