 ots of college kids talk about “safe spaces” these days, where
they can be free from dangerous ideas like “freedom of speech.” But in
the real world, a safe space is a place where you don't get killed. Here are a few
examples.
ots of college kids talk about “safe spaces” these days, where
they can be free from dangerous ideas like “freedom of speech.” But in
the real world, a safe space is a place where you don't get killed. Here are a few
examples.
Walk-in freezers
Everybody knows the risk of getting trapped in a cold room, which is typically at 4°C. But I once worked in a lab that had a walk-in freezer, kept at −20°C. They used it to store, for some reason, big jars of E. coli bacteria. There was no light inside and no intercom. To get to it, you went through the cold room, opened the heavy sliding door, and gingerly stepped across the icy floor. You always told somebody where you were; if you got trapped, you were locked behind two layers of windowless, soundproof doors, and nobody would ever find you.
(If this ever happens to you, and there's no alarm button, jam a metal rod in the cooling fans. The heat exchanger will frost up, warming up the room and setting off an alarm. Or just breathe on the temperature sensor if it's exposed.)
Ultracentrifuge rotors
An ultracentrifuge is a big, $50,000 machine about the size of a washing machine. Unlike a modern washing machine, an ultracentrifuge is inherently dangerous (though not as dangerous, I suppose, as those old-fashioned wringer washers, which had two pinching rollers driven by a powerful geared-down motor).
A typical rotor was up to a foot in diameter, weighed 20 pounds, and it spun at 40,000 rpm. The rotor is held in place only by gravity. At those speeds (considered low today) the surface of the rotor was moving at 768 miles/hour, or just over the speed of sound, so the centrifuge is designed not to start up unless there's a good vacuum in the chamber.
At 1127 ft/sec, if that vacuum goes, the aerodynamic forces could lift the rotor off the spindle. Or if it's unbalanced by more than a few grams on one side, it will start to resonate at certain speeds, and can be flung off. Or if it develops a crack it can fall apart at high speed. The vacuum chamber is about 1/2 or 3/4 inch thick seamless stainless steel, as is the lid, and they're designed to contain the rotor if that happens.
We didn't have an ultracentrifuge, so I used to visit a neighboring lab to use theirs. One day I came in and found that somebody had crashed the rotor. The heavy stainless steel lid and the chamber were massively dented and twisted.
A Ti70 rotor weighs 13 pounds. At its maximum speed of 70,000 rpm, the edge experiences a force of 450,000×g, making its effective weight 5,850,000 pounds, or about 12 times the weight of an EMD SD70ACe-T4 locomotive engine. That sounds like a lot, but it's misleading: the kinetic energy is what's important, not the weight.
Using the formula
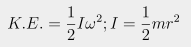
where ω is radians per minute and I = moment of inertia (for a rotating solid disk,
where m=mass and r=radius), gives you 174,582 Joules of energy. That's equivalent
to a locomotive falling 7.8 centimeters, a Toyota Corolla falling 13.99 meters,
or the rotor itself being dropped from 1.87 miles.
Even that calculation is a bit misleading, because a rotor would probably bounce around for a while, releasing its energy more slowly than a falling Toyota. Whether a half inch of 316 stainless steel can contain it or not, it's clear that a supersonic 13-pound titanium projectile can be hazardous, and I can attest that it can also create quite a mess.
Modern ultracentrifuges are a lot smaller, but the rotors spin at up to 120,000 rpm, and some are rated for up to 1,019,000×g, so their angular velocity is still over the speed of sound. There's a big sticker inside that warns you to push down the button that locks the rotor in place. If you forget, you're reminded by a loud clunking sound, and by a loud beeping alarm that goes off—and nowadays they shut off automatically.
Electricity
For some reason, when I was a kid people used to give me their old vacuum-tube radios. Unlike modern-day electronic stuff, the old stuff was easy to fix: you could just solder in a new capacitor or whatever was broken. But they were hazardous. They had a metal chassis that served as the ground, and it was directly wired to the 120 volt line current.
There were no polarized plugs in those days, so if you plugged the radio in one way it was safe. If you plugged it in the other way, you had live current on every metal part of the radio. If you had high skin resistance, you could tell which way it was plugged in by dragging your finger lightly across a metal part.
If they wanted me dead, they should have tried harder. They missed their chance.
Of course, 120 VAC is nothing compared to really high voltage. I once built a little helium-neon laser power supply, which created pulses of 7,000 volts. Watching that He-Ne tube suddenly change from a fuzzy pink neon-like glow to a focused, collimated beam as you turned up the voltage was one of the coolest things ever. It was powered by a little 9-volt battery, but I made the circuit board too small, not realizing how important it is to keep the components far apart. At 7kV, electricity will jump across the air by a centimeter or so, so that power supply lit up with little sparks jumping from one component to another. The dragging-your-finger trick doesn't work, either: if you get too close, the electricity arcs across to you, making a distinctive snapping sound—followed by a distinctive ”Ouch!” sound.
This reminds me of my misadventure with a tantalum capacitor, where I powered up a circuit and left the room for a second, at which point there was a very loud bang and the capacitor suddenly ceased to exist. Luckily I was not there, or I'd be deaf now.
Mercury
You might think pharmacologists would all be experts on toxic chemicals. In my school that wasn't the case. I was the only biochemist taking pharmacology lab, and they had a special room where the glass manometer was kept. There were drops of mercury spilled everywhere. I was standing in the back, and casually pointed out to a fellow student that mercury liquid turns into vapor, and in a closed space it will accumulate and become highly toxic. The professor overheard me, noticed the mercury, and quickly ushered everyone out. And some grad student was in big trouble.
There are lots of other risks in doing science, including X-rays, toxic chemicals, and pathogenic viruses. Then there's liquid nitrogen: I know one small company where a technician lost an eye from an exploding improperly stored vial. Of course, there are other professions that are far more dangerous than biochemistry. Being in the military is one. Even being a plumber is dangerous, from what the guy who repaired my sewage line tells me: people pour large amounts of Drano in the line, and then ‘forget’ to let the plumber know about it.
You can try to protect yourself from human stupidity, but if you're protected from the real world, you start to lose your perspective about what's dangerous and what's not. It's no coincidence that the scientific fields that make the most accurate judgements about risk are the most respected. It's also probably why those who deal with real, dangerous phenomena think that letting kids turn into snowflakes may be the most dangerous thing of all.
dec 10, 2017, 5:34 am; calculations tweaked 10:14 am; last edited dec 11 2017, 7:24 am
