 or the past several weeks, the Internet has been buzzing about how ivermectin, a drug
given to dogs for heartworm and to cattle for grubs, might be a cure for
Covid-19.[1] But now scientists at Nuventra, a small pharma consulting
company in North Carolina, have thrown cold water on it.
or the past several weeks, the Internet has been buzzing about how ivermectin, a drug
given to dogs for heartworm and to cattle for grubs, might be a cure for
Covid-19.[1] But now scientists at Nuventra, a small pharma consulting
company in North Carolina, have thrown cold water on it.
You may recall that researchers found a 5000-fold reduction in viral mRNA after 48h. But in pharmacology, dose is everything.
Ivermectin is approved in the USA for onchocerciasis (river blindness) and strongyloidiasis (an intestinal parasitic disease) in humans. The concentration needed to knock down SARS-CoV-2 in the test tube (in vitro) was 5 μM. The new study[2] says this is more than 35 times higher than the maximum lung concentration obtainable when the approved dose of ivermectin, 200 μg/kg, is given orally. To inhibit the virus just by 50% would require 2 μM concentration. To reach 5 μM, you'd have to give enormous amounts of ivermectin.
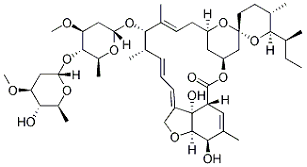
Ivermectin structure
Ivermectin has a wide safety margin compared to chloroquine, but temporary blindness and fatal encephalopathy have occurred, and the FDA has issued a warning about using it for Covid-19.
This illustrates a quandary for clinical researchers. To test a drug, you'd want to give each subject the same dose. But in the real world, even though patients may vary by a factor of three in body weight, they all get the same size pill. In one clinical trial I was involved with, people argued endlessly whether the dose, calculated for the patient's weight, should be 20 or 25. It was ridiculous, because in a clinical trial the clinical staff just give all the patients the same amount of drug, even though their weights varied by two-fold. Their reasoning makes sense: in the real world, the patient is never going to titrate their dose.
This factor also seems to rule out chloroquine as an antiviral drug because a safe dose for one person might be fatal to another. The ratio of the toxic dose to the effective dose, known as the therapeutic index, is only 3 for chloroquine. That means if you weigh 33% less than average and accidentally take one extra dose, you could be pushing up daisies.
If the Nuventra scientists are right (and unlike epidemiological models most pharmacokinetic models aren't too bad), it's one more reason why we ought to be skeptical of clinical trial results. Some groups have reported positive results with ivermectin. But what ivermectin tells us is that we need basic research to discover how it's working and then to measure whether it's actually doing what it's supposed to. Often we find it's doing something entirely different.
Ivermectin is supposed to act by opening the glutamate-gated chloride ion channels in invertebrate nerve and muscle cells. This lets more chloride into the cells, which paralyzes the parasite. That can't explain how it would work against a virus, which doesn't have any ion channels. Caly et al. speculate that ivermectin binds to importin-α/β1, a protein that transports the virus into the cell's nucleus, and prevents it from binding the viral protein.
But the finding is only good as a starting point for drug development, which means that scientists will be testing thousands of variations of ivermectin on the computer before synthesizing the most potent ones and testing them on cells. And hoo boy, is that software expensive. Looking at ivermectin's chemical structure (see image), synthesizing it is not an enviable task. (Merck originally did it. Supposedly a better synthesis has been devised, but Nature wants money to read about it.)
Whenever anyone tells you that a certain drug or combination of drugs does something in the test tube, the first thing to ask is whether it reaches the concentration needed to be effective in the patient. Medicinal chemists know a drug faces many obstacles before it gets to where it's needed. And there are lots of bad things it can do to you on its way there.
Maybe the FDA needs a more flexible system for approving drugs on a temporary basis, but it looks like approving ivermectin would be a waste of time.
1. Caly, L., Druce, J., Catton, M., Jans, D., Wagstaff, K. (2020). The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir Res 178, 104787. https://doi.org/10.1016/j.antiviral.2020.104787 https://www.sciencedirect.com/science/article/pii/S0166354220302011
2. The Approved Dose of Ivermectin Alone is not the Ideal Dose for the Treatment of COVID-19 Schmith, V. D., Zhou, J., Lohmer, L. R. MedRxiv pre-proof, Apr 26 2020. 10.1101/2020.04.21.20073262
apr 26 2020, 6:57 am
