|
chemistry
How to Make a Substitute for Lysol 4 in 1 Spray Disinfectantby T Nelson |

|
chemistry
How to Make a Substitute for Lysol 4 in 1 Spray Disinfectantby T Nelson |

|
by T Nelson |
Lysol® Four In One™ Disinfectant was the best cleaner ever made for removing soap scum on glass shower doors. You just sprayed it on the glass and rinsed, and the soap scum disappeared. I liked this stuff so much I ordered a case for the lab, because it turned out it was also good for removing stains caused by culture media on stainless steel biosafety cabinets, also known as tissue culture hoods.
4 in 1 disinfectant was an acidic cleaner, so it worked much better than products like Tilex, which is a mixture of EDTA and nonionic detergents.
Unfortunately, Reckitt Benckiser Inc., the makers of Lysol, changed the formula and the product no longer works. No other product on the market suitable for casual use comes close. The few remaining bottles are being sold on the Internet for 85 bucks, and I was so desperate I almost bought one.
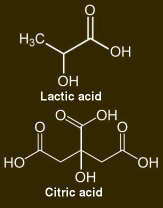
But ... wait a minute ... I almost forgot ... I'm a chemist! So I experimented with various formulations to make a substitute. The only active ingredient listed on the bottle is lactic acid, 4%. However, neither lactic acid nor citric acid by themselves worked very well, even at concentrations up to 10%. This can only mean one thing: the label is wrong.
Clearly there's some kind of organic solvent in there, along with a yellow dye, a thickener, and a lemon odor, possibly lemon oil, which is probably there to cover up the faint but unpleasant smell of lactic acid. I tried mixing lactic acid with Lysol® Power & Fresh™ Multi-surface© cleaner with Waterfall Splash & Mineral Essence™ Scent, but it didn't work, probably because it wasn't acidic® enough.
Rbnainfo.com gives the composition as:
| Ingredient | Function |
|---|---|
| Water | Makes it wet |
| Lactic acid | Makes it acidic |
| Dipropylene glycol butyl ether | Organic solvent |
| C9-11 alcohols ethoxylate | Detergent |
| Sodium C14-17 alkyl sec sulfonate | Detergent |
| Sodium hydroxide | Makes it less acidic |
| Fragrance | Covers up the stench of the other ingredients |
| Acid yellow 3 | Turns it yellow |
According to the MSDS, the pH is only 3, and there's no indication why they would have discontinued it. It says “Firefighters should wear full protective clothing including self contained breathing apparatus.” I think this would probably be overdoing it, but I guess it's better to be safe than sorry.
Here is the final composition. It works almost as well as Lysol™®© 4 in 1. I would much rather buy some, but you do what you gotta do.
Citric acid could also be used, and chemically it should work better, because the three carboxylic acid groups in citrate are great at chelating calcium. It also smells nicer, and you can, apparently, according to the Amazon.com reviewers, sprinkle it on your food. But citric acid makes the floor squeak if you spill it on your linoleum tile.
Acetic acid / vinegar also works, but leaves a bad smell. Don't use muriatic acid (hydrochloric acid); it would corrode metal and cause burns. Remember that acid should never be mixed with hypochlorite (Clorox) bleach, or it will release chlorine gas, which is harmful to your lungs.
Have fun, use at your own risk, don't try to sell it, don't drink it, and whatever you do don't spray this stuff in your eyes. It would probably hurt like hell.
Update Lactic acid has a terrible odor, so I made a liter of this stuff using citric acid instead (use about 50 to 100 grams per liter; dissolve it before adding the detergent and alcohol). It works amazingly well. Hard water stains just fall off the glass and rinse away. But watch out, it is slippery on the floor.
aug 01, 2014; updated may 02, 2016