Alzheimer's disease and cholesterol
This page contains some extra, less-technical material from my forthcoming book chapter on Alzheimer's disease and cholesterol. The book was published in late 2006.
Incidence of Alzheimer's disease
Alzheimer's disease (AD) is the most common form of dementia in aging. By age 85, between 25 and 47% of the population will suffer from this devastating disease. The exact percentage is a matter of some dispute, with some researchers saying that 50% of the population will get Alzheimer's disease by age 80, and others placing the number closer to 35%. In one study, researchers calculated the following set of probabilities:
| Age bracket | Incidence | 95% confidence limits |
| 65-74 | 3% | 0.8 - 5.2% |
| 75-84 | 18.7% | 13.2 - 24.2% |
| >85 | 47.2% | 37.0 - 63.2% |
Whatever the exact numbers, it's clear that the risk increases almost exponentially with age. One of the tragedies of Alzheimer's disease is that it deprives people of their awareness, thinking ability, and memory during their later years, when they should be enjoying their retirement.
Risk factors in Alzheimer's disease
In addition to age, several other risk factors have been found that increase the chances of getting Alzheimer's disease. As with risk factors for cancer, having a risk factor for Alzheimer's disease increases the risk, but it is impossible to predict whether any specific individual will get the disease. The risk factors include head trauma, genetic factors, high cholesterol, and atherosclerosis.
Most cases of Alzheimer's disease are idiopathic, which means that there is no known cause, genetic or otherwise. Some scientists have questioned whether cholesterol and head trauma are risk factors for Alzheimer's disease. The data are not 100% conclusive.
Head trauma
There is some evidence that severe head injury increases the risk of Alzheimer's disease later in life. One theory why this might be so is that brain cells react to injury by trying to extend new axons and dendrites. There is some suggestion that this process might make use of amyloid precursor protein (APP), a large protein that is often found in the growing tips of neurons where it may participate in synaptogenesis (the formation of new synapses) or cell adhesion. Somehow, after the brain is injured in a head trauma, the process goes wrong, and (according to one theory) a chain of events is set into motion that ultimately ends in Alzheimer's disease.
This theory is similar to the prevailing theory about how carcinogens cause cancer. Many toxic substances can cause cancer not by causing mutations, but by putting stress on the cell. In these cases it's not the toxin itself that causes cancer, but the cell's attempt to divide and repair the damage. Somehow the repair process goes horribly wrong, resulting in catastrophe. (This is an oversimplified version of the theory, which actually involves many other components).
Axons, which are the "output leads" of neurons (which act as tiny switches or microchips in the brain), are especially affected. In the brains of Alzheimer's patients there are millions of small structures called "neurofibrillary tangles", made up primarily of a protein named tau. Tau is a major structural component of axons, where it is involved in microtubule assembly. These microtubules are giant protein structures responsible for transporting other molecules from the cell body to the end of the axonal filament.
The normal function of APP is unknown. Some researchers have suggested that APP may be involved in memory. If anti-APP antibodies are injected into the brains of chickens, it blocks memory formation. Whatever its function, there is clearly an important role for APP in normal synaptic plasticity and neuronal growth.
Head trauma also can cause the release of blood into the brain. Blood contains large amounts of iron, which can be very toxic to brain cells. However, iron is not believed to be a factor in Alzheimer's disease.
Genetic mutations
A small percentage of patients have "familial Alzheimer's disease", which is caused by inherited changes in one of three proteins:
- Amyloid precursor protein
- Presenilin 1
- Presenilin 2
Another risk factor is Apolipoprotein E. Apolipoprotein E is an important cholesterol carrying protein. It is found on the outer surface of low-density lipoproteins, or LDL, which are small particles that float in the blood plasma and carry cholesterol to cells that need it. The apolipoprotein E acts as a targeting protein. It binds to specific LDL receptors and the LDL particles are taken up into the cell, where the cholesterol is released. Although there are also apolipoprotein E receptors at the blood-brain barrier, LDL uptake into the brain from blood plasma is not a significant source of the brain's cholesterol. Most of the cholesterol in the brain is made by the brain cells as they need it, and any extra cholesterol in the brain is oxidized and rapidly removed.
Cholesterol and atherosclerosis
Having high cholesterol and atherosclerosis may also be a risk factor. Since high cholesterol can produce atherosclerosis, it is difficult to know whether the cholesterol, the atherosclerosis, or some other factor downstream of both of them is responsible.
Notice that almost all of the major risk factors for Alzheimer's disease, with the exception of head trauma, involve cholesterol in one way or another. Besides apolipoprotein E, amyloid precursor protein also interacts with cholesterol. APP is a large protein in the brain that is cut into smaller pieces by proteases known as secretases. These products are small protein chunks or peptides. There are three types of secretases:
- Alpha-secretase (the "good" secretase)
- Beta-secretase (the "bad" secretase)
- Gamma-secretase (the "ugly" secretase)
Beta- and gamma-secretase work together to cut APP into a peptide known as beta-amyloid. Beta-amyloid can be 40, 42, or 43 amino acids long. It is found in high levels in Alzheimer's disease plaques. Beta-amyloid is a very toxic peptide. Concentrations of this peptide as low as 1 nM, which is about 38 molecules per cell (if the cell is a small one), are sufficient to kill a neuron. Beta-amyloid has binding sites for, and binds to, both cholesterol and copper. As shown in the figure below, the "good" secretase (alpha-secretase) cuts APP in the middle of the region where beta-amyloid would be formed. This prevents that particular molecule of APP from being converted into beta-amyloid. However, for whatever reason, alpha-secretase does not react with beta-amyloid. So once beta-amyloid is formed, it is free to do whatever it does to the cell.
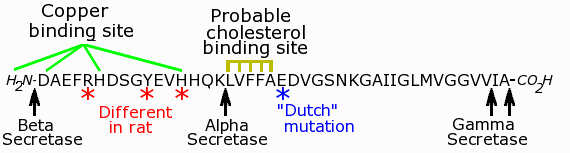
Features of the beta-amyloid peptide. Each letter stands for a different amino acid. The cleavage sites of the secretases are shown. This peptide is only a small portion of the parent APP molecule, which has 770 amino acids, compared to only 40, 42, or 43 in beta-amyloid. The 42-amino acid version is shown here.
Notice that alpha-secretase cuts APP right between the cholesterol-binding site and the copper-binding site (see figure above). This may be one of the reasons the product created by alpha-secretase is relatively harmless. One theory is that beta-amyloid binds to copper and cholesterol, and converts cholesterol into a new, oxidized molecule. When the copper and cholesterol binding sites are separated, beta-amyloid can no longer keep copper and cholesterol in close enough proximity to react, and therefore can no longer oxidize cholesterol.
In the process of reacting with cholesterol, beta-amyloid also creates hydrogen peroxide, which is rapidly detoxified by other enzymes in the cell. Some researchers have proposed that hydrogen peroxide may account for some of the oxidative stress seen in cells, proteins, and lipid molecules from Alzheimer's patients. However, others have pointed out that although large amounts of hydrogen peroxide can be toxic, many enzymes produce small amounts of hydrogen peroxide normally; at the concentrations found in the cell, hydrogen peroxide may act more like a signaling molecule than a toxin. In the same way, nitric oxide and carbon monoxide are deadly poisons in high concentrations, but act as signaling molecules in the cells that produce them.
In fact, beta-amyloid can create two completely different forms of oxidized cholesterol, depending on the conditions. One form is highly toxic to neurons, while the other is harmless. Researchers do not yet understand the conditions that favor one product over the other. (This topic is discussed in much greater technical detail in my chapter!)
Aluminum
At one time, it was thought that aluminum might contribute to Alzheimer's disease. Of course, there are many types of aluminum:
- Aluminum oxide, which is biologically inert
- Salts, such as aluminum chlorohydrate or aluminum sulfate
- Inorganic compounds, such as aluminum chloride (AlCl3, which reacts rapidly with water when anhydrous)
- Metallic aluminum
The possible involvement of metals is interesting, because many metals bind to proteins and can cause them to aggregate. However, the aluminum theory of Alzheimer's has been largely dismissed because little or no aluminum can be found in amyloid plaques. A possible role for copper and possibly zinc is still being investigated by many scientists. Copper is particularly intriguing, because both APP and beta-amyloid are copper-binding proteins.
Difficulties for researchers
Despite the fact that Alzheimer's is currently a "hot topic", as far as diseases go, researchers in the field frequently complain about how hard it is to study Alzheimer's disease. There are many reasons why finding a cure for Alzheimer's disease is difficult.
The first reason is that Alzheimer's disease does not occur naturally in animals. Like Parkinson's disease, Alzheimer's disease occurs only in humans. Although some animals, like dogs, can have dementia (loss of brain cells) in their old age, they do not contract Alzheimer's disease. The closest Alzheimer's-like phenomena occur in aged nonhuman primates such as orangutans, rhesus monkeys, and chimpanzees, which sometimes get brain lesions containing beta-amyloid. Another species, the lemur Microcebus murinus also gets senile plaques in its old age. But none of these are the same as full-blown Alzheimer's disease.
Another reason is that the beta-amyloid molecule has a different amino acid sequence in humans. For example, the beta-amyloid in rats and mice differs at three positions from that of humans (see figure). One of these amino acid substitutions prevents rat beta-amyloid from binding to copper. Another occurs very close to the point where beta-amyloid binds to cholesterol. Yet these differences, important as they may be, are not sufficient to explain why guinea pigs and rabbits, whose beta-amyloid is identical to that of humans, do not get Alzheimer's disease. Other factors, such as diet must also be important. Rabbits, for example, are strict vegetarians, and have no source of dietary cholesterol. If rabbits are put on a diet containing cholesterol, they become sick as dogs (so to speak). According to some reports, structures resembling senile plaques also begin to appear in their brains.
Because of these problems, it is necessary for researchers to use tissue samples from human patients who have died from the disease. As might be expected, these tissue samples are quite difficult to obtain, and many researchers are forced to use mouse models in which the normal mouse amyloid precursor protein has been replaced with a human form of the protein. Mutant mice carrying human APP die at an early age with symptoms that resemble Alzheimer's disease in some ways; but using these mice assumes that Alzheimer's disease is caused by, or at least fundamentally related to, beta-amyloid. This is a dangerous assumption, and makes the mice useless to researchers interested in discovering what causes Alzheimer's disease.
Why is it so difficult to obtain human tissue? The brain is what makes us an individual, and relatives who would readily donate part of their liver or blood may not like the idea of giving up such a critical part of their loved one. Part of it is also political. During the early part of the AIDS epidemic several years ago, on many occasions we observed hundreds of AIDS activists demonstrating outside our laboratory, essentially begging us to perform experiments on them. (Of course we declined, because that would have been unethical.) Although we felt great sympathy for them, some of us couldn't help thinking that what these activists really wanted was to be able to participate in the pathological activities that caused their disease without having to pay the terrible price. Whatever their motivation, AIDS activists now have all of Hollywood and the pop music industry supporting their cause. Alzheimer's disease patients, although they are in much greater number than AIDS patients, have no such advocacy groups. But even if sufficient human autopsy samples were available, finding the causes of a disease ten or twenty years later, when the pathological changes relevant to the disease are hopelessly mixed with nonspecific changes caused by cell death, inflammation, and failed attempts by the remaining cells to adapt, is no easy task.
There are many other diseases similar to Alzheimer's disease, including fronto-temporal dementia and motoneuron disease inclusion dementia (MNDID). These are almost as common as Alzheimer's disease, but there is even less public awareness of these diseases. Because of the seeming lack of interest in these diseases, it is often difficult for researchers in these diseases to get funded and published, and even more difficult to obtain specimens to study. Thus, not many scientists can afford to study these diseases.
How could cholesterol cause Alzheimer's disease?
Most people (well, most chemists anyway) think of cholesterol as a fairly harmless and inert molecule. We eat large amounts of it in our diet. It is essential for the life of all cells, including those in the brain; in fact, the brain is two percent cholesterol by weight. How could such an abundant and unreactive molecule be harmful?
It just so happens that we have a perfect example of the problems that can be caused when there is too much cholesterol in the cell. It is Niemann-Pick disease type C, a disease very much like Alzheimer's disease. The cause of Niemann-Pick type C is well understood. It is caused by a genetic defect in one of two proteins, named NPC1 and NPC2. When these proteins are mutated, they lose their ability to transport cholesterol from one point to another inside the cell. Cholesterol then accumulates in the cell. The result is a form of dementia that shares many of the same pathological characteristics of Alzheimer's disease, including tau-containing neurofibrillary tangles and beta-amyloid plaques. Not only that, but mutations in apolipoprotein E, which are a risk factor for Alzheimer's disease, also predispose patients for Niemann-Pick type C. Of course, there are differences between the two diseases as well. But the remarkable similarities of these two diseases illustrate that cholesterol by itself can produce symptoms very similar to those seen in Alzheimer's disease.
Statins
This is not to say that patients should immediately run out and start taking statins to lower their cholesterol. The evidence on whether statins are beneficial, while encouraging, is still incomplete. The question of whether cholesterol is involved in Alzheimer's disease at the biochemical level and the question of whether high serum cholesterol is a risk factor are two completely different things. It is also important to remember that the cholesterol theory of Alzheimer's disease is still just one of several theories. The theory about the importance of cholesterol oxidation is still relatively new. Some scientists dispute that high serum cholesterol is a even a risk factor for Alzheimer's disease. Yet even if dietary cholesterol should turn out not to be a factor in Alzheimer's disease, there is clearly something going on between amyloid precursor protein, beta-amyloid, and cholesterol. The question is ... what?
