What Causes Alzheimer's Disease?
Theories of Alzheimer's disease
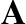 lzheimer's disease is a devastating and mysterious illness that strikes
people with increasing frequency as they age. In the earliest stages of
Alzheimer's disease, patients lose synapses, which are connections in the
brain between neurons that are essential for learning and memory. At the
biochemical level, many of the molecules involved in Alzheimer's are also
involved in normal memory. Therefore, understanding Alzheimer's disease
will also give us clues about how memory works, and vice versa.
lzheimer's disease is a devastating and mysterious illness that strikes
people with increasing frequency as they age. In the earliest stages of
Alzheimer's disease, patients lose synapses, which are connections in the
brain between neurons that are essential for learning and memory. At the
biochemical level, many of the molecules involved in Alzheimer's are also
involved in normal memory. Therefore, understanding Alzheimer's disease
will also give us clues about how memory works, and vice versa.
There are actually two types of Alzheimer's disease: familial or "early onset" Alzheimer's disease and sporadic or "late onset" Alzheimer's disease. The only difference, as far as we know, is that one form is inheritable, or familial, and the other is not. The symptoms of familial Alzheimer's disease often begin when the patients are in their mid-fifties. This form of Alzheimer's disease can be inherited from your parents, but it is rare. The vast majority of cases are sporadic Alzheimer's disease, which is not hereditary. In these cases, symptoms are rarely detectable before age 65, and there are no inherited mutations, as far as we know, that could explain why they are sick.
In practical terms, this means that if your parents and grandparents did not have early-onset Alzheimer's disease, and you are experiencing memory failures, it is very unlikely to be an early symptom of Alzheimer's unless you are at least 65 years old.
In both familial and sporadic Alzheimer's disease, the patients have amyloid plaques, which are tiny clumps of aggregated protein, and neurofibrillary tangles, which are disorganized remnants of neurons, in their brain. The plaques are composed primarily of a small protein called beta-amyloid (Aβ, pronounced "A beta" /eɪ 'beɪtə/, sometimes called amyloid-beta). Scientists do not know whether Aβ and the deposits of Aβ cause Alzheimer's disease, or are the body's way of protecting against it.
Alzheimer patients usually also have a condition known as cerebral amyloid angiopathy, where Aβ is deposited extracellularly in the walls of blood vessels in the brain. Angiopathy means that there is some relationship between Alzheimer's disease and cardiovascular problems. Indeed, stroke is a risk factor for Alzheimer's disease.
In this article, I will discuss the current theories and epidemiological results that might explain the molecular basis of sporadic Alzheimer's disease, and explain their strengths and weaknesses.
Cellular mutation
One theory is that a change in DNA--perhaps a mutation--causes Alzheimer's disease. The word 'mutation' has two different meanings to biologists: genetic or germ line mutations, which are inherited, and cellular or somatic mutations, which are not. Cancer is caused by cellular mutations: one single cell develops a mutation somewhere in its DNA that causes that cell to continue dividing indefinitely. These mutations cannot be passed down to the patient's children. But all the cells that descended from the original cell do have the mutation. These cells form a tumor.
 3-D Structure of beta-amyloid 1-42 [5]. The starting point (N-terminal) is
at the left. The yellow atom is a methionine group, which is believed
to be important for toxicity.
3-D Structure of beta-amyloid 1-42 [5]. The starting point (N-terminal) is
at the left. The yellow atom is a methionine group, which is believed
to be important for toxicity.
So far, no one has observed any cellular mutations in Alzheimer's disease, either in cellular DNA or in mitochondrial DNA [8] (which is separate from regular DNA). In familial Alzheimer's disease, which is about 3-5% of patients, the cause of Alzheimer's disease is known. It is a genetic mutation in one of three proteins: presenilin 1, presenilin 2, or amyloid precursor protein. These mutations cause the cell to produce large amounts of Aβ and to produce a different, more toxic form of Aβ known as Aβ1-42.
What if there were a cellular mutation in the sporadic Alzheimer's disease patients that affected these same proteins? Unfortunately, that theory doesn't work, because neurons don't divide. The mutation would be stuck in one single cell. It might work if a large percentage of neurons happened to spontaneously acquire the same mutation, but this seems unlikely. The only possibility would be a mutation that produces a transmissible substance, like a prion.
Interestingly enough, some researchers have found that Aβ binds very strongly to prion protein [2]. What's more, the prion protein has a high affinity for oligomerized Aβ--the toxic form (see below)--but not normal Aβ. When researchers created a strain of mice that lacked prion protein, the harmful effects of oligomerized Aβ on LTP (a phenomenon similar to learning) were abolished. This strongly suggests that oligomerized Aβ affects learning by binding to prion protein.
Not only that, but traumatic brain injury increases the levels of prion protein in the brain by over three fold [3]. One possible explanation for this is that the prion protein might be involved in synaptogenesis, which is part of the brain's repair mechanism. Synaptogenesis is also important for memory.
However, Alzheimer's disease is not a prion disease; the symptoms are quite different. Cells from Alzheimer's disease patients have no vacuolization, as would occur in a prion disease, and there is no deposition of abnormal prion protein in Alzheimer's disease. Also, unlike Alzheimer's disease, prion diseases do not produce inflammation. Nevertheless, the prion link is very interesting (or as they say on Star Trek, "fascinating") to Alzheimer researchers.
Accumulation of injury-related molecules
Another theory is that accumulation of toxic protein molecules might cause Alzheimer's disease. This theory is probably the most widely held one. According to this theory, an excess of Aβ, or some other molecule, is produced by some unknown mechanism, perhaps as the result of a brain injury. Or, the enzyme that breaks down the molecule stops working. As a result, the molecule continues to accumulate until it reaches a concentration where it becomes toxic. At this point, it starts killing neurons.
Some scientists believe that tau protein is the culprit in Alzheimer's disease. Tau is a microtubule-associated protein in the axons that helps maintain their linear shape. In Alzheimer's, tau becomes hyperphosphorylated, which causes the paired helical filaments, which are made of tau, to become tangled. Other scientists are of the opinion that tau hyperphosphorylation is a secondary effect, perhaps caused by Aβ.
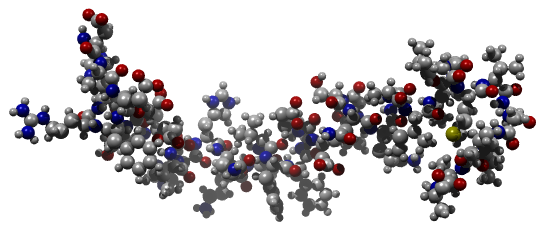
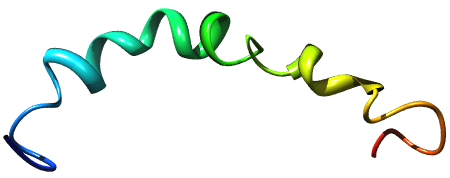 Ribbon structure of beta-amyloid 1-42 [5]
Ribbon structure of beta-amyloid 1-42 [5]
For Aβ, there's an additional wrinkle. When concentrations of Aβ reach a certain level, the Aβ molecules bunch up and form dimers, trimers, and larger oligomers. It turns out that these oligomerized forms of Aβ are much more toxic to neurons than pure Aβ. In fact, if you took some pure Aβ and put it on some cells in a culture dish, you would need to use a concentration of over 20 micromolar--a huge concentration for a protein--to kill the cells. Not enough to be crushing the cells by its weight, but close. If you put oligomers on the cells, it only takes 5 micromolar [10]. This is still an awful lot, but it's getting closer to the concentration that is found in the brains of some Alzheimer's disease patients. So this theory sounds plausible.
However, a four-fold improvement is not much. If the oligomer is a tetramer and it's four times as toxic as a monomer, the actual toxicity per monomeric unit is unchanged. So in this case, oligomerization is not doing anything. Researchers make oligomers by dissolving Aβ a certain way. The way you dissolve your Aβ that you buy from the store makes a huge difference in how toxic it is. Oligomers made by different labs, using almost exactly the same procedure, vary by more than 28,000-fold in toxicity. Clearly, there's something going on here that we don't understand.
To see why the whole Aβ theory should be accepted with caution, consider an example from physics. Physicists sometimes say that they would like to create mini-black holes in their supercollider. This scares the heck out of people (as it's probably intended to do). So the physicist's public relations director has to stand up and point out that the physicist was only kidding, and if it were that easy to create a black hole, there would be black holes popping up everywhere. We would all be dead. Likewise with Aβ. This theory needs to explain why Aβ would suddenly start to accumulate. If it were that easy to get Alzheimer's disease, everyone would have it. And we would all be dead.
Interestingly, Aβ is not increased in the blood plasma of Alzheimer patients. In the CSF, it is actually decreased. So whatever is causing the buildup of Aβ in brain is not systemic, but is specific to the brain or the blood-brain barrier.
Just recently, scientists have found that we can eliminate Aβ from the brain by immunizing people against it. Immunizing patients creates antibodies that practically suck all the Aβ out of patients' brains. Unfortunately, early results seem to indicate that this has absolutely no effect on the course of the disease.
This is very surprising, because it suggests that Aβ and the plaques that are formed from it might be only an epiphenomenon. But in that case, how can we explain how the mutations in familial Alzheimer's, whose only apparent effect is to increase Aβ, cause the disease? One possibility might be that by mutating Aβ, the cell somehow messes up its ability to protect against the real culprit. Or maybe a by-product of Aβ, not Aβ itself, is causing the damage.
Vicious circles
Many researchers have postulated that Alzheimer's disease might result from some sort of vicious circle. For example, it is known that traumatic brain injury increases the levels of Aβ in the brain. Perhaps Aβ in turn causes oxidative stress, which occurs when oxidizing molecules such as superoxide react with and destroy other, important molecules. One such important molecule is neprilysin, which is an enzyme that normally degrades Aβ. If neprilysin is destroyed, Aβ will accumulate. Thus, according to the theory, a small injury could, in some patients, produce a cascade of more and more Aβ.
The problem with vicious circle theories is that you have to explain why the brain doesn't recover from it. All biomolecules in every cell are turned over, or recycled, continuously. They are degraded and re-synthesized all the time. Not only that, but the cell has some very clever mechanisms of ensuring that it creates exactly the amounts of each protein that are needed, no matter what. This prevents vicious cycles from being created. For example, in the neprilysin example above, if you proposed that neprilysin gets destroyed, you would have to explain why the cell doesn't simply produce more and more of it to compensate. Nature "knows" about vicious circles, and invented elaborate biochemical machinery to prevent them.
Of course, it is possible that a large excess of some toxic biomolecule could overwhelm these regulatory mechanisms, or sabotage them in some way. But most biochemists don't like this idea, because Alzheimer's disease takes a long time to get started. Vicious circles are usually very short-lived. The cell either recovers from them or it dies. There are only two sure ways to produce a long-term change in the cell without killing it: by changing its DNA or by introducing a new, exogenous molecule that can't be degraded.
Age-dependent decrease in a critical biomolecule
One way DNA changes is by the natural aging process. As a cell ages, some genes, which are active parts of the DNA molecule, are turned off, and others are turned on, according to a pre-set program. This is why we grow old. It's possible that, in some people, one or more genes are turned off earlier than in other people.
We can see this effect every day. Some people become bald sooner than others. In some people, the hair turns white, while in others, it retains its original color for many years. Everyone ages at a different rate.
This is the most pessimistic theory of Alzheimer's disease, because it implies that Alzheimer's disease is a normal part of aging. If so, it might even be a mistake to call Alzheimer's disease a disease. If we are all genetically programmed to get Alzheimer's disease as a normal part of getting old and dying, it will be very tough to cure it.
It has been pointed out that aging per se is probably not a cause of Alzheimer's, because no form of progeria causes Alzheimer's, and early-onset Alzheimer patients do not experience abnormal aging.
Virus, toxin, or bacteria
The two golden rules in biochemistry are:
1. All biomolecules turn over.
2. There are no bad biomolecules.
Aβ is a biomolecule, and it's produced naturally by the brain.
If it didn't have some beneficial purpose, it wouldn't be there.
Yet many researchers are trying to discover ways to get rid of it.
A friend of mine who works on Parkinson's disease tells me the same
thing is happening in that field with regard to synuclein. Scientists
are trying to find a way to get rid of the protein synuclein. But
synuclein, like Aβ, isn't a bad molecule; it's just misunderstood.
What is the normal function of Aβ? Aβ is produced in the brain when it is injured: after head trauma, known as TBI or traumatic brain injury, or after hypoxia. There is some suggestion that in patients experiencing severe traumatic brain injury, those patients who produce more Aβ have a greater chance for survival.
Here are some of the functions that Aβ might participate in:
- Synaptogenesis--Aβ might play an important role in memory or recovery from head injury by inducing the growth of new synapses.
- Natural tranquilizer, protecting against excitotoxicity after injury.
- Mopping up iron or hemoglobin after head trauma. When the brain is injured, small amounts of blood leak into the brain. The hemoglobin in blood is highly toxic to neurons. Aβ strongly binds to heme, which is the iron-carrying part of hemoglobin.
- Blood coagulation promoter. The brain might have an extra mechanism to stop bleeding without creating large blood clots.
- Protection against oxidants. In many diseases, there are large increases in oxidized molecules, such as 4-hydroxynonenal, acrolein, or 7β-hydroxycholesterol. These molecules are highly toxic to brain cells. Some researchers believe that Aβ can act as an antioxidant, which means it can convert these toxic oxidized molecules to something else, or even prevent them from being formed.
- Protection against copper or zinc. Aβ binds copper and zinc. In fact, Aβ binds to a great many things, including cholesterol, growth factor receptors, and other molecules of Aβ. Researchers have found that Aβ interacts with dozens of other kinds of molecules. This makes it hard to figure out which interaction is the most important.
We can speculate that maybe evolution has made a tradeoff: greater chance of survival after traumatic brain injury in exchange for a miserable old age of dementia, loss of memory, and an early death. But so far, it's only speculation.
In fact, because of the recent failures of anti-Aβ therapies, many researchers have come to doubt that Aβ causes Alzheimer's disease. But if not Aβ, what are the alternatives? So far, searches for infectious agents, their DNA, or serum antibodies (which would indicate a past infection) in Alzheimer's patients have been negative [6, 7]. Some viruses can produce neurofibrillary tangles. A few other diseases, such as diabetes, Parkinson's disease, and mad cow disease, produce amyloid deposits. But these are chemically quite different from those found in Alzheimer's disease, because they do not contain Aβ. So even if it's not the cause, it seems clear that Aβ is somehow involved in Alzheimer's disease.
But even if Alzheimer's disease is caused by excess Aβ, why does the brain suddenly go hog-wild, allowing tons of it to accumulate in some patients? Aβ, and the molecules that produce and degrade it, continuously turn over. So, if Aβ is accumulating, unless there is something wrong with the patient's DNA, there must be an external force, like a pathogen, that is interfering with its normal metabolism. So far, no one has found any virus, toxin, or any other pathogen that can cause Alzheimer's disease. No one has ever been able to take an extract from an Alzheimer's disease brain and induce Alzheimer's disease in an animal. The closest that anyone has come is to get it to convert normal amyloid into aggregated fibrils [9]. But even this only worked in specially engineered transgenic mice that were guaranteed to get Alzheimer's-like symptoms. Furthermore, in that experiment, synthetic Aβ, pure synthetic Aβ oligomers, and mixtures of the two didn't work at all. Only Aβ extracts from a real patient worked. This suggests that there was something else in the extract from that patient's brain besides Aβ that was causing the effects.
In fact, the inability to produce Alzheimer's in mice should not be surprising, because only humans can get Alzheimer's disease. Even our transgenic mice do not really have Alzheimer's. And consider this:
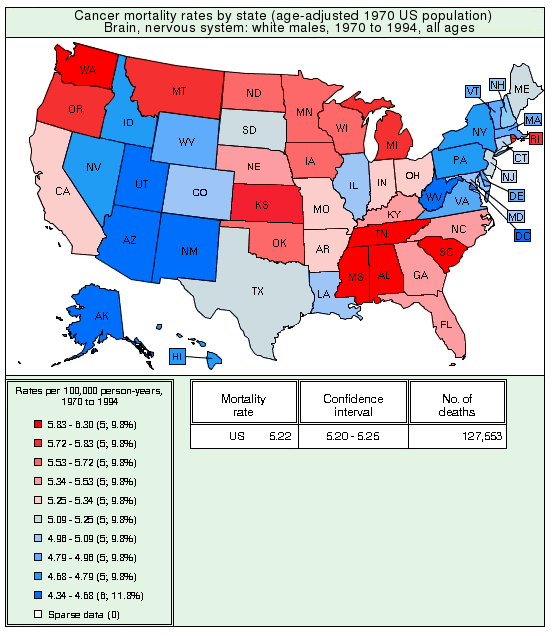
| State | Rate | State | Rate | State | Rate | State | Rate |
|---|---|---|---|---|---|---|---|
| Tennessee | 36.2 | Oklahoma | 28.1 | West Virginia | 23.2 | Massachusetts | 19.8 |
| Washington | 35.9 | South Dakota | 27.3 | California | 23.2 | D of Columbia | 19.1 |
| Louisiana | 34.2 | Kansas | 27.2 | Arkansas | 22.6 | Pennsylvania | 18.9 |
| Alabama | 33.2 | Texas | 27.2 | Wyoming | 22.7 | Rhode Island | 18.8 |
| South Carolina | 32.4 | Georgia | 27.0 | Minnesota | 22.5 | Florida | 18.4 |
| Arizona | 31.3 | Mississippi | 26.7 | Virginia | 22.5 | New Mexico | 18.3 |
| North Dakota | 29.8 | New Hampshire | 26.1 | Wisconsin | 22.4 | New Jersey | 17.6 |
| North Carolina | 29.5 | Ohio | 26.0 | Nebraska | 21.8 | Maryland | 17.5 |
| Idaho | 29.4 | Vermont | 25.7 | Utah | 21.5 | Nevada | 17.1 |
| Maine | 29.1 | Missouri | 25.4 | Alaska | 21.3 | Connecticut | 16.1 |
| Kentucky | 28.9 | Iowa | 25.4 | Michigan | 21.2 | Hawaii | 11.4 |
| Oregon | 28.9 | Indiana | 24.7 | Illinois | 20.8 | New York | 9.2 |
| Colorado | 28.5 | Montana | 23.9 | Delaware | 20.0 |
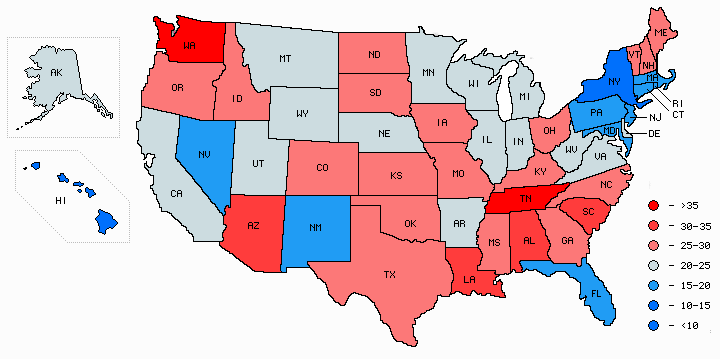
Age-corrected geographical distribution map of Alzheimer's death rates by state
Graph drawn by Mapmaker utility based on data from the Centers for Disease Control (CDC).
These numbers, compiled by the National Center for Health Statistics at the CDC, are the deaths per 100,000 population, adjusted for age. The death rate is 3.9 times higher in Tennessee than in New York. You don't have to be an epidemiologist to see that this table is strong evidence for an environmental cause of Alzheimer's disease. ('Environmental' means a factor outside the individual, such as a virus, lifestyle factor, dietary factor, food toxin, or water contaminant.)
Earlier statistics from the CDC showed Montana, Utah, and Vermont as the highest, with age-adjusted Alzheimer's rates of 56, 52, and 50 deaths per 100,000, with New York still the lowest at 12.1---a ratio of 4.6 to 1 [4].
If anyone can figure out what is different about Tennessee and Washington compared to Hawaii and New York, I'd be interested in hearing about it. My email address is at the top of the page. Here's a place to start: http://www.epa.gov/triexplorer/geography.htm
Correlation of Alzheimer's disease with depression
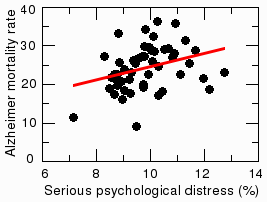
Depression in adults shows no significant correlation with Alzheimer's among the fifty states. This is because cultural, ethnic, and economic factors play such an important role in depression. You would probably not be surprised to find that adults in places like West Virginia, Nevada, and Utah, where there are no palm trees or sunny beaches, are more likely to be depressed, while people in Hawaii are the least. These cultural effects swamp out any correlation with Alzheimer's disease. However, depression in elderly people is highly correlated with Alzheimer's [14, 15]. Both depression and Alzheimer's cause a loss of synapses, or neuron-neuron connections in the brain. This loss of synapses might partially explain the depression in Alzheimer patients. However, the psychological impact of receiving an Alzheimer diagnosis is undoubtedly also a factor.
I should point out that there's a lot of bad data out there on this topic. Just recently, one group published a survey claiming that rates of depression are higher in some states than others. But upon examination, it turned out that their definition of “depression” was actually a measure of how well the state met the political agenda of the survey-takers.
A related measure known as 'serious psychological distress' also correlates with Alzheimer's. According to data from the National Survey on Drug Use and Health (NSDUH), the rates of serious psychological distress among persons aged 18 or older are highest in West Virginia, Rhode Island, and Utah, and lowest in Hawaii and South Dakota. The coefficient of correlation with Alzheimer's was 0.32, which is statistically significant at the level of p=0.019. This means there was only a one in 52 chance that the correlation was a coincidence. Distress doesn't correlate well with other diseases, like brain cancer. So it seems unlikely that the distress could result just from learning they have Alzheimer's. Could it be that psychological trauma in early adulthood influences whether a person gets Alzheimer's later in life? Some researchers say a stress-filled life increases the risk [16, 17], but it is not yet generally accepted. More research is needed on this topic.
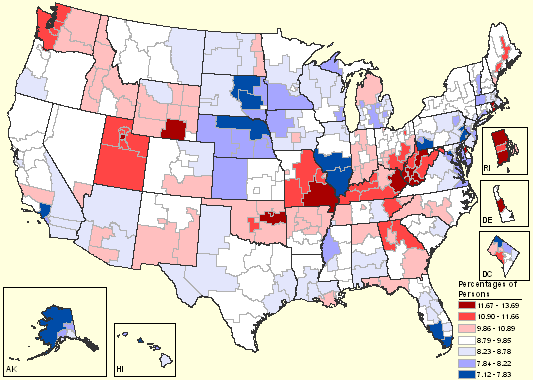
Map of serious psychological distress among persons 18 and older. This graph was created by the Substance Abuse and Mental Health Services Administration (SAMHSA), Office of Applied Studies, a branch of the US Department of Health and Human Services. The graph is based on the National Survey on Drug Use and Health, 2004 and 2005. (http://www.oas.samhsa.gov/substate2k6/MH.htm)
Correlation of Alzheimer's disease with diabetes and environmental factors
Diabetes is a known risk factor for Alzheimer's disease. The correlation between Alzheimer's and diabetes is highly significant at p=0.0032 (see figure below, left). Obesity, another known risk factor, also correlated weakly with Alzheimer's disease (p=0.021, not shown).
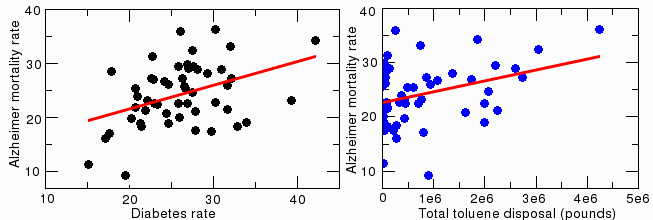
Correlation between Alzheimer's disease and diabetes
There is no significant correlation between Alzheimer's disease and several other lifestyle-related factors that might affect brain function, including asthma, autism, exercise, sexually transmitted diseases, fluoride, lead or copper in drinking water, alcohol intake, or tooth loss. Levels of a variety of atmospheric and water pollutants (based on data from the EPA website), including cadmium, mercury, thallium, vanadium, silver, zinc, arsenic, nickel, selenium, aluminum, benzene, acrylonitrile, radon, and carbon disulfide also do not correlate with Alzheimer's disease. There was a weak correlation for toluene (figure above, right). It is statistically significant (p=0.016), but the result is unconvincing because so many states had zero toluene, so it is probably a statistical artifact.
One word of caution, though: since these are state-wide statistics, and not individual or "longitudinal" studies, there is no guarantee that the individuals who get Alzheimer's are the same as the people who get diabetes. The same is true for the other statistics reported here. A more thorough investigation is needed to prove that any two diseases are really correlated. We can't even begin to determine the cause of the disease from comparing its prevalence in different states. But the state-wide correlations done here do give us a starting point--a hint about what to look for.
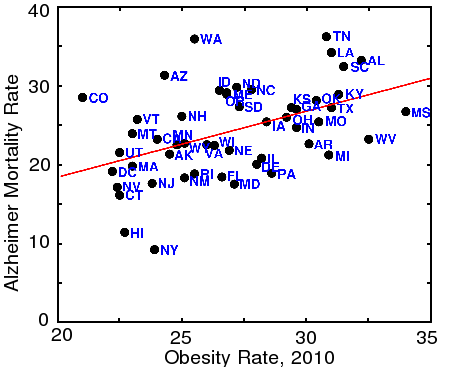
Correlation between Alzheimer's disease and Obesity
States with the highest incidence of obesity (BMI>30) also tend to have higher rates of Alzheimer's disease, as shown by the above graph. Many factors, including race, culture, and obviously diet, also affect obesity, but the correlation with Alzheimer's is highly significant (p=0.0005, or 1 in 1900). (Data from the CDC)
Another predisposing factor is major surgery, such as coronary artery bypass surgery. Major surgery with anesthesia seems to put a tremendous amount of stress on the brain. This causes post-operative cognitive decline, a well-documented form of transient cognitive impairment that generally resolves in a few months. Some evidence suggests that this can also be a risk factor for Alzheimer's later in life. However, the results are not clear, because most studies so far have not clearly distinguished Alzheimer's from other types of dementia.
Correlation of Alzheimer's disease with cancer
The two states with the highest Alzheimer rates (Tennessee and Washington) are also among the states with highest rates of cancer of the brain and nervous system. The figure below shows the age-corrected 1970-1994 rates per 100,000, taken from the National Cancer Institute ( http://cancercontrolplanet.cancer.gov/atlas/index.jsp ). These numbers are for white males, which means any differential effects of race or gender are eliminated.
Although there are a couple of outliers (notably Arizona, Louisiana, and Rhode Island), the states with the highest Alzheimer rates also tend to have the highest rates of brain cancer. The five highest states for brain cancer are Tennessee, South Carolina, Mississippi, Washington, and Alabama. Other types of cancer don't show a positive correlation with Alzheimer's disease; in fact, for some types of cancer, such as thyroid cancer, there appears to be a weak protective effect.
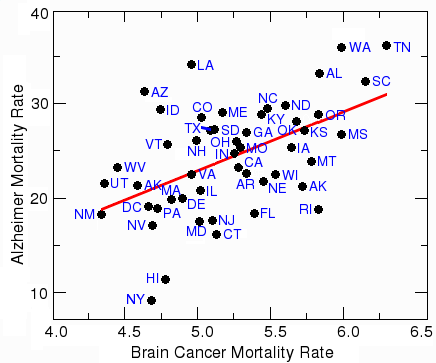
Correlation between brain cancer and Alzheimer's disease
The correlation between Alzheimer's and brain cancer is statistically highly significant, with a probability (p value) of 0.00018, which means there is a one in 5,500 chance that this correlation is due to chance. The most likely explanation is that a standard treatment for brain cancer is whole-brain irradiation. Dementia, or loss of healthy neurons, is a common side-effect of ionizing radiation directed at the head. Another factor is that many chemotherapeutic agents are neurotoxic, which means they kill healthy neurons. This is especially true for Alzheimer patients, where the blood-brain barrier is sometimes compromised. It is likely that treatment of brain cancer causes dementia that is either mistaken for Alzheimer's disease or increases its severity.
The protective effect of the other types of cancer is probably caused by the tendency of pathologists to list a single cause of death at autopsy. If a patient with both Alzheimer's and cancer dies, the pathologist will frequently list cancer, and not Alzheimer's, as the cause of death [11]. One reason for this is that the standard death certificate form (PHS T-003) only has a single line for the immediate cause of death [12]. However, a small number of studies that did not rely on death certificates still found a small protective effect of cancer [13]. In fact, one group of researchers [18] has raised the interesting possibility that cancer and Alzheimer's are opposites: in cancer, there is too much growth, while in Alzheimer's, there is not enough. If this is true, a cure for one might also be a cure for the other. (However, even if it is true, it does not mean you can cure yourself of Alzheimer's by getting cancer!)
We need your help
The cause of late-onset Alzheimer's disease is still a mystery. But this is one mystery that the general public can help us solve. There must be something that patients with Alzheimer's have in common--maybe something that happened to them when they were younger, or something they ate that was unusual. We need to know what that is. If you have some anecdotal evidence to share, tell your doctor, or contact us directly. Or you can email me, using the address at the top of the page.
References
[1] http://www.alz.org/alzheimers_disease_facts_figures.asp; data from Kung, HC; Hoyert, DL; Xu, JQ; Murphy, SL. Deaths: Final Data for 2005 National vital statistics reports. Vol. 56, no. 10. Hyattsville, Md.: National Center for Health Statistics; 2008.[2] Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM.
[3] Marciano et al., J. Neurosci 24, 2866, 2004.
[4] Mortality trends for Alzheimer's disease, 1979-1991. Vital and Health Statistics 20(28).
[5] Created using Chimera using data from the following paper: The alpha-to-beta Conformational Transition of Alzheimer's Abeta-(1-42) Peptide in Aqueous Media is Reversible: A Step by Step Conformational Analysis Suggests the Location of beta Conformation Seeding Tomaselli, S., Esposito, V., Vangone, P., van Nuland, N.A., Bonvin, A.M., Guerrini, R., Tancredi, T., Temussi, P.A., Picone, D. (2006) Chembiochem 7: 257-267
[6] Age Ageing. 1987 Sep;16(5):311-4. A sero-epidemiological study of conventional infectious agents in Alzheimer's disease. Renvoize EB, Awad IO, Hambling MH.
[7] Brain. 1987 Aug;110 ( Pt 4):907-15. A study of viral genomes and antigens in brains of patients with Alzheimer's disease. Pogo BG, Casals J, Elizan TS.
[8] Trends Neurosci. 2008 May;31(5):251-6. The mitochondrial impairment, oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Fukui H, Moraes CT.
[9] Meyer-Luehmann, M. et al. Science 313, 1781-1784 (2006).
[10] J Neurosci. 2006 May 31;26(22):6011-8. Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. Deshpande A, Mina E, Glabe C, Busciglio J.
[11] Trade-off between cancer and aging: what role do other diseases play? Evidence from experimental and human population studies. Yashin AI, Ukraintseva SV, Akushevich IV, Arbeev KG, Kulminski A, Akushevich L. Mech Ageing Dev. 2009 Jan-Feb;130(1-2):98-104.
[12] Stallard, E., 2002. Underlying and multiple cause mortality at advanced ages: United States 1980-1998. North Am. Actuarial J. 6 (3), 64-87.
[13] Neurology. 2005 Mar 8;64(5):895-8. Alzheimer disease and cancer. Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC.
[14] Fortschr Neurol Psychiatr. 2009 Depressive Disorders in Dementia and Mild Cognitive Impairments: Is Comorbidity a Cause or a Risk Factor? Preuss UW, Siafarikas N, Petrucci M, Wong WM.
[15] Int Rev Psychiatry. 2008 Aug;20(4):382-8. Depression in Alzheimer's disease: phenomenology, clinical correlates and treatment. Starkstein SE, Mizrahi R, Power BD.
[16] Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA. Neurology. 2005 Jan 25;64(2):380-2.
[17] Chronic distress and incidence of mild cognitive impairment. Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Neurology. 2007 Jun 12;68(24):2085-92.
[18] Behrens MI Lendon C Roe CM 2009. A common biological mechanism in cancer and Alzheimer's disease? Cur Alz Res 6, 196-204.