 n 1998, Bill Clinton demolished the al-Shifa pharmaceutical plant in Sudan with
missiles, claiming it was producing VX nerve agent. That claim was based
on a single soil sample identified by the CIA as containing EMPTA, or O-Ethyl
methylphosphonothioic acid, said to be a chemical precursor for making VX.
n 1998, Bill Clinton demolished the al-Shifa pharmaceutical plant in Sudan with
missiles, claiming it was producing VX nerve agent. That claim was based
on a single soil sample identified by the CIA as containing EMPTA, or O-Ethyl
methylphosphonothioic acid, said to be a chemical precursor for making VX.
Before that, there was the 1981 yellow rain incident, in which a yellow material was found in Laos and South Vietnam. The US government claimed was a trichothecene mycotoxin dropped by the Soviet Union, but government labs in Britain, France, and Sweden were unable to replicate the findings.
Now the British government has accused Russia of poisoning former spy Sergei Skripal with Novichok, which is the generic name of a number of organophosphorus chemical weapons invented by the Soviet Union during the Cold War.

A typical triple quadrupole mass spectrometer (left) and liquid chromatography system (center).
Those of us in the sciences are used to seeing hard evidence for any scientific claim. Governments are known to make mistakes—we still remember that aspirin factory—so skepticism is warranted, especially when a mistake could lead to international conflict.
How do we know this time it's true? Out of idle curiosity, yesterday at lunch I looked up a few technical articles in the open literature to see how laboratories might identify nerve agents. It turns out that the US CDC, of all places, has developed some clever new ways. The Japanese had the unfortunate opportunity to acquire some actual victim samples from a terrorist incident on their soil a few years back, and they have a few articles as well.
I read Vil Mirzayanov's book State Secrets (reviewed here) when it was first published, and there's a little bit of technical data in it, including chemical formulas, though it's not clear which of these the UK is claiming to have found. (On March 19 the BBC quoted Russia's UK ambassador Alexander Yakovenko as suggesting that it was A-234.) The information needed to make them is not spelled out in Mirzayanov's book. He did, however, provide enough information that an expert could deduce their properties.
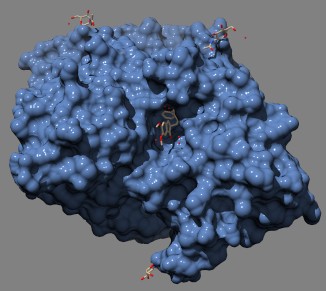
3D model of acetylcholinesterase complexed with donepezil (Aricept). Donepezil (brown structure in center of image) fits into a crevice in the much larger AChE protein molecule from Pacific electric ray (shown in blue). (protein = Kryger et al.(1999) Structure Fold. Des. 7: 297–307 Source)
Even to a nonspecialist it's immediately evident that they're highly toxic molecules. No one in their right mind would try to synthesize them. In the book Mirzayanov relates how some of his fellow scientists, highly trained and equipped with the latest equipment, tragically and horribly died trying to analyze them. He called his lab a sharashka, a prison laboratory.
VX is insanely toxic: it irreversibly inhibits AChE, or acetylcholinesterase, the enzyme that breaks down the neurotransmitter acetylcholine. But many drugs, including aspirin, which inhibits cyclooxygenases, are also irreversible inhibitors. The reason nerve agents are so deadly is the target: AChE is on the postsynaptic membrane of cholinergic nerve terminals, and if you block it the nervous system goes into a literal frenzy that rapidly kills the victim.
Some AChE inhibitors are used clinically; tacrine and donepezil reversibly inhibit AChE and provide some symptomatic benefit in Alzheimer's disease.
There are generic bioassays and enzyme assays, such as Ellman's method, where you add acetylthiocholine and use DTNB, which turns yellow, to measure the free thiocholine that's produced. This tells you how much AChE activity remains in the blood sample of a victim.
That doesn't tell you what the inhibitor is, so typically they'd measure the agent's breakdown products in the blood or urine. That's tricky, because as a small molecule its breakdown products are fairly ordinary. Nowadays, the standard instrument for doing that is the triple quadrupole mass spectrometer. When combined with a chromatography system, either liquid chromatography (LC) or gas chromatography (GC), you get high confidence that you're measuring what you think you are.
Like the CRM-114 discriminator in that Kubrick movie, a triple quad LCMS is designed not to give any signal at all . . . unless the molecule has three different properties that are exactly right. If not, you don't see any signal. If you do, and if there's enough sample, you'd then fragment the molecule and compare its fingerprint to the one in your database. It has to match perfectly, or it's not the right molecule.
For instance, below is a typical fragmentation spectrum. It shows the parent, or starting material (cholesterol in this case), which has a mass of 386, along with a lot of smaller fragments.
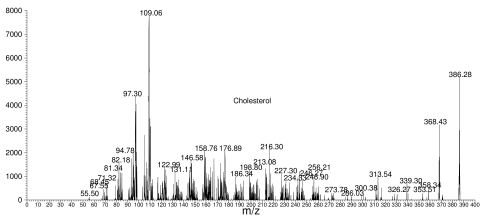
Cholesterol fragmentation spectrum
The numbers in the spectrum (368.43, 109.06, etc.) represent masses of various fragments, and they tell the chemist a great deal about what is in the molecule. However, it's easy to get fooled, because some molecules are so similar that their fragmentation patterns are almost identical.
It's also common to get “ghost” peaks left over from a previous sample, or peaks for similar stuff that has bits in common with what you're looking for. So the analyst has to run many controls to make sure it's not a false identification. I've seen beginners make this mistake many times (though not, I hasten to add, with toxins—the chemicals we study are naturally occurring molecules, and they're a lot safer. Even the CDC doesn't keep those toxins around; they have tiny amounts of them shipped in from government-affiliated labs).
It's likely that nobody knows much about the metabolism of these chemicals—the MSDS in Hank Ellison's Handbook of Chemical and Biological Warfare Agents is maddeningly vague—but their hydrolysis and decomposition products are easy to predict. With nerve agents, there's also a time limit: as the sample ages, it gets slowly hydrolyzed by the enzyme. The products are phosphonic acids and sulfides.
An improved method is to measure the agent that's stuck to human serum albumin, or HSA, a blood protein. The advantage is that sample aging doesn't happen when it's on HSA.
Given that (1) everybody knows the structure and (2) the degradation products, and that (3) we can detect them at the part per trillion level, and (4) the symptoms of organophosphorus poisoning are unmistakable, it's safe to ask why in the world anyone would use them. There can only be one reason: they want us to know who did it.
It strikes me that, given the power of these instruments, if Ms May and Mr Trump want us to believe them, they'd release the lab results to the world, or at the very least tell us how, technically, they can be so sure what the toxin is. If you're going to risk a war over a chemical identification, you'd better be sure you're right—and that your citizens believe you. Porton Down's credibility is on the line. They should insist on releasing the data.
If Putin wanted the West to believe him, his blanket denial was exactly the wrong thing to say. No one is going to trust a Russian lab in a case like this. If Russia were truly innocent, Putin would have promised to launch an investigation, even if only to find a scapegoat. The only reasonable conclusion is that the Russians wanted the other spies to know they are next.
The stupidity and arrogance of the Russian government, if they are really behind this, is mind-boggling. On the other hand, if Porton Down is wrong, it would be very embarrassing for the UK.
Update (Mar 31, 2018): Does the UK need a a sample to identify A-234?
Given that authentic samples of these molecules are no doubt a PITA to keep around, the question arises: could they identify it without having access to the molecule?
The answer is a definite maybe:
- If the structure is known (as it is for A-234), a high-resolution mass spectrometer like an FT-ICR could give you the chemical formula, which is the number of C,H,N,O,F, and P atoms in the molecule, if the molecule is reasonably small. It doesn't identify the compound, but it narrows down the possible structures dramatically.
- Commercial and public-domain software can predict a fragmentation pattern from an organic molecule with reasonable accuracy. The reverse—identifying a small molecule from MS/MS data alone—is still difficult. A review by Scheubert et al. discusses techniques such as CID voltage ramping that improve the accuracy.
- Without a standard sample, the chromatography step is not possible, and the identification is much less certain.
However, it would not pose a great difficulty for an experienced chemical weapons chemist to create a small sample for analysis.
Update Tim Hayward has additional information here. He claims that the US and UK governments are concealing information about the A-230 series of compounds. He also says the fragmentation spectrum of A-234 was included in the NIST mass spectral library but has since been deleted. There is nothing sinister about this: the US government has consistently tried to make it as difficult as possible for terrorists to produce dangerous substances.
Hayward also expresses skepticism about why Skripal and his wife were heavily sedated and unable to communicate. Again, there is nothing mysterious about this: a medically induced coma would be an effective way of preventing brain injury that would result from overstimulation from these agents. He is saying that Novichok may have additional modes of toxicity besides AChE inhibition. There are hints from other sources that this could be the case.
Update (Apr 10, 2018): Fake experts
Lew Rockwell's site has an article quoting a “former Director of the U.S. Army's Depleted Uranium Project and an expert on chemical weapons, with a PhD in health physics” as disputing the conclusion that Novichok was found:
it is pure b.s.
It is impossible to put liquid nerve agent on a door knob without trashing the entire area 100 200 meters and more radius for a long time. weeks months or more.
The only partially useful antidote is mark 1 kit- atropine, diazapam [sic], prodoxyaline [sic] chloride.. no good on vx tabun soman novachuk [sic] multiple 7 .
Anyone who went near this would go down anyone who touched them would go down.
Aside from the technical errors, any expert on chemical weapons would know how to spell pralidoxime and diazepam, two of the most well known drugs in the field. He would certainly know how to spell Novichok, and he'd know the old saying that the dose makes the poison.
Another well known news site claims that five milliliters—a huge amount for such a toxic chemical— of this stuff would have been needed. Beware of fake experts.
Update (Apr 14, 2018): BZ
RT reports that Russian Foreign Minister Sergey Lavrov is now claiming that the Spiez Laboratory in Switzerland has identified the poison used on Skripal as 3-quinuclidinyl benzilate (BZ).
The structure, molecular mass, chemical formula, and chemical properties of BZ are quite different from A-234. It would be very difficult to confuse them. Its pharmacological effects are also opposite to AChE inhibitors. BZ is a muscarinic acetylcholine receptor blocker, which means that, like atropine and scopolamine, it could theoretically act as an antidote to an AChE inhibitor. Instead of producing miosis (constriction of the pupil) it would produce mydriasis. Blood tests would easily discriminate between these two agents.
The US Government book says that when BZ is administered topically (e.g., through hand contact) its effects are delayed by approximately 24h. It is said to cause delirium, stupor, mumbling, disrobing behavior, and coma. The antidote for BZ is physostigmine, a cholinesterase inhibitor.
Spiez Laboratory couldn't have made this big an error. BZ must be in his bloodstream. The only explanation is that the Brits are using BZ as an antidote. It makes sense: BZ isn't the bogeyman hallucinogen the press would have us believe. It's about 3–4 times as potent as scopolamine, but more long-lasting. It's not in the textbooks, but then neither is Novichok.
mar 28 2018, 5:18 am. last updated apr 14 2018, 4:56 pm. edited for clarity apr 15, 4:57 am
